Microbial Communities and Methane Cycling in Bromeliad Tanks and Leaf Axils of Oil Palms”
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Growing Alcantarea
Bromeliaceae VOLUME XLII - No. 3 - MAY/JUNE 2008 The Bromeliad Society of Queensland Inc. P. O. Box 565, Fortitude Valley Queensland, Australia 4006, Home Page www.bromsqueensland.com OFFICERS PRESIDENT Olive Trevor (07) 3351 1203 VICE PRESIDENT Anne McBurnie PAST PRESIDENT Bob Reilly (07) 3870 8029 SECRETARY Chris Coulthard TREASURER Glenn Bernoth (07) 4661 3 634 BROMELIACEAE EDITOR Ross Stenhouse SHOW ORGANISER Bob Cross COMMITTEE Greg Aizlewood, Bruce Dunstan, Barry Kable, Arnold James,Viv Duncan, David Rees MEMBERSHIP SECRETARY Roy Pugh (07) 3263 5057 SEED BANK CO-ORDINATOR Doug Parkinson (07) 5497 5220 AUDITOR Anna Harris Accounting Services SALES AREA CASHIER Norma Poole FIELD DAY CO-ORDINATOR Ruth Kimber & Bev Mulcahy LIBRARIAN Evelyn Rees ASSISTANT SHOW ORGANISER Phil Beard SUPPER STEWARDS Nev Ryan, Barry Genn PLANT SALES Pat Barlow Phil James COMPETITION STEWARDS Dorothy Cutcliffe, Arnold James CHIEF COMPETITION STEWARD HOSTESS Gwen Parkinson BSQ WEBMASTER Ross Stenhouse LIFE MEMBERS Grace Goode OAM Peter Paroz, Michael O’Dea Editors Email Address: [email protected] The Bromeliad Society of Queensland Inc. gives permission to all Bromeliad Societies to re- print articles in their journals provided proper acknowledgement is given to the original author and the Bromeliaceae, and no contrary direction is published in Bromeliaceae. This permission does not apply to any other person or organisation without the prior permission of the author. Opinions expressed in this publication are those of the individual contributor and may not neces- sarily reflect the opinions of the Bromeliad Society of Queensland or of the Editor Authors are responsible for the accuracy of the information in their articles. -

Multi-National Conservation of Alligator Lizards
MULTI-NATIONAL CONSERVATION OF ALLIGATOR LIZARDS: APPLIED SOCIOECOLOGICAL LESSONS FROM A FLAGSHIP GROUP by ADAM G. CLAUSE (Under the Direction of John Maerz) ABSTRACT The Anthropocene is defined by unprecedented human influence on the biosphere. Integrative conservation recognizes this inextricable coupling of human and natural systems, and mobilizes multiple epistemologies to seek equitable, enduring solutions to complex socioecological issues. Although a central motivation of global conservation practice is to protect at-risk species, such organisms may be the subject of competing social perspectives that can impede robust interventions. Furthermore, imperiled species are often chronically understudied, which prevents the immediate application of data-driven quantitative modeling approaches in conservation decision making. Instead, real-world management goals are regularly prioritized on the basis of expert opinion. Here, I explore how an organismal natural history perspective, when grounded in a critique of established human judgements, can help resolve socioecological conflicts and contextualize perceived threats related to threatened species conservation and policy development. To achieve this, I leverage a multi-national system anchored by a diverse, enigmatic, and often endangered New World clade: alligator lizards. Using a threat analysis and status assessment, I show that one recent petition to list a California alligator lizard, Elgaria panamintina, under the US Endangered Species Act often contradicts the best available science. -

Diversity and Levels of Endemism of the Bromeliaceae of Costa Rica – an Updated Checklist
A peer-reviewed open-access journal PhytoKeys 29: 17–62Diversity (2013) and levels of endemism of the Bromeliaceae of Costa Rica... 17 doi: 10.3897/phytokeys.29.4937 CHECKLIST www.phytokeys.com Launched to accelerate biodiversity research Diversity and levels of endemism of the Bromeliaceae of Costa Rica – an updated checklist Daniel A. Cáceres González1,2, Katharina Schulte1,3,4, Marco Schmidt1,2,3, Georg Zizka1,2,3 1 Abteilung Botanik und molekulare Evolutionsforschung, Senckenberg Forschungsinstitut Frankfurt/Main, Germany 2 Institut Ökologie, Evolution & Diversität, Goethe-Universität Frankfurt/Main, Germany 3 Biodive rsität und Klima Forschungszentrum (BiK-F), Frankfurt/Main, Germany 4 Australian Tropical Herbarium & Center for Tropical Biodiversity and Climate Change, James Cook University, Cairns, Australia Corresponding author: Daniel A. Cáceres González ([email protected]) Academic editor: L. Versieux | Received 1 March 2013 | Accepted 28 October 2013 | Published 11 November 2013 Citation: González DAC, Schulte K, Schmidt M, Zizka G (2013) Diversity and levels of endemism of the Bromeliaceae of Costa Rica – an updated checklist. PhytoKeys 29: 17–61. doi: 10.3897/phytokeys.29.4937 This paper is dedicated to the late Harry Luther, a world leader in bromeliad research. Abstract An updated inventory of the Bromeliaceae for Costa Rica is presented including citations of representa- tive specimens for each species. The family comprises 18 genera and 198 species in Costa Rica, 32 spe- cies being endemic to the country. Additional 36 species are endemic to Costa Rica and Panama. Only 4 of the 8 bromeliad subfamilies occur in Costa Rica, with a strong predominance of Tillandsioideae (7 genera/150 spp.; 75.7% of all bromeliad species in Costa Rica). -

The Effects of Human Disturbance on Vascular Epiphyte in the Brazilian Atlantic Forest
The effects of human disturbance on vascular epiphyte in the Brazilian Atlantic Forest Edicson Parra-Sanchez Imperial College London Department of Life Science Silwood Park Campus Thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy (PhD) April 2018 1 “What we have to practice today is solidarity, one must not approach people to say Here we come to give the charity of our presence, to teach our science, to show you your mistakes, your ignorance, your lack of basics. We go with investigative zeal, and humble spirit, to learn in great source of wisdom which is the people.” Che Guevara 2 Declaration The data collection and work presented in this thesis is all my own. Dr Cristina Banks-Leite did provide guidance throughout my thesis. Chapters are organized as papers, and I used “we” to recognize the role of my supervisor Dr Cristina. Data sources and software are referenced along the text. The support of different people is acknowledge as follows: Data collection was done with field assistants Jordy Jerez, Manon Czuckermand and Tiago Gloria. The study design was done by ECOFOR (Biodiversity and Ecosystem Functioning in Degraded and Recovering Amazonian and Atlantic Forests). Taxa identification was done with the help of MSc Gabriel Marcusso (Piperaceae), Dr Carlos Nunes and Eric Hagsater (Orchidaceae), Ms Suzana Martins and Marcio Leofegario (Bromeliaceae), Dr Alain Chautems (Gesneriaceae), and Thomas Croat (Ferns). In chapter 2, advice on the model fitting and interpretation was provided by Dr Jack Hatfield. In chapter 3, advice on the interpretation of the n-dimension analysis was provided by Dr Thomas Guillerme (as author of the “dispRity” R package). -

A New Species of Epimorius Feeding on Bromeliaceae in Costa Rica (Lepidoptera: Pyralidae: Galleriinae)
28 SOLIS: A New Epimorius from Costa Rica TROPICAL LEPIDOPTERA TROPICAL LEPIDOPTERA, 11(1-2): 28-32 (2003) A NEW SPECIES OF EPIMORIUS FEEDING ON BROMELIACEAE IN COSTA RICA (LEPIDOPTERA: PYRALIDAE: GALLERIINAE) M. ALMA SOLIS Systematic Entomology Laboratory, PSI, ARS, USDA, National Museum of Natural History, MRC 168, Washington, DC 20560-0168, USA asolis @ sel.barc.usda.gov ABSTRACT.- Epimorius maylinae n. sp., reared from Werauhia gladioliflora (Wendland) (Bromeliaceae) in Costa Rica, is described. The pupa is also described and compared to the pupa of Epimorius testaceellus Ragonot, a species which also has been reared from Bromeliaceae. Athaliptis Schaus, described from Costa Rica, is newly synonymized with Epimorius. Epimorius cymonia Schaus, n. comb., is the type species of Athaliptis and is reported for the first time from Pitcairnia sp. (Bromeliaceae). Other galleriines reared from Bromeliaceae are discussed. KEYWORDS: Alpheias, Athaliptis, biology, Bromeliaceae, Cacotherapiini, Central America, Epimorius maylinae n.sp., Genopaschia, hostplants, immatures, Mesoamerica, Neotropical, Panama, pineapple, pupa, Tirathabini, taxonomy. Fig. 1-2. Adults of Epimorius maylinae Solis, n. sp.: 1) allotype female (forewing length 18mm), left; 2) holotype male (forewing length 18mm), right. Five species now comprise Epimorius: E. suffusus (Zeller), the poor condition. Another female specimen collected by Schaus and type species E. testaceellus Ragonot, E. prodigiosus Whalley, E. Barnes from Sixola River, Costa Rica was dissected by D. C. caribellus Ferguson, E. cymonia (Schaus), and E. maylinae Solis, Ferguson (USNM slide #57,332), but was misidentified as E. the new species described in this study. In 1991, Ferguson reviewed suffusus (Ferguson, 1991). Comparison of these two slides, genitalia the genus Epimorius Zeller. -

An Alphabetical List of Bromeliad Binomials
AN ALPHABETICAL LIST OF BROMELIAD BINOMIALS Compiled by HARRY E. LUTHER The Marie Selby Botanical Gardens Sarasota, Florida, USA ELEVENTH EDITION Published by the Bromeliad Society International June 2008 ii INTRODUCTION TO EDITION XI This list is presented as a spelling guide for validly published taxa accepted at the Bromeliad Identification Center. The list contains the following information: 1) Genus number (the left-hand number) based on the systematic sequence published in the Smith & Downs monograph: Bromeliaceae (Flora Neotropica, number 14, parts 1-3; 1974, 1977, 1979). Whole numbers are as published in the monograph. 2) Species number (the second number) according to its systematic position in the monograph. Note: Taxa not included in the monograph or that have been reclassified have been assigned numbers to reflect their systematic position within the Smith & Downs framework (e.g., taxon 14.1 is related to taxon 14). The utility of this method is that one may assume for example that Tillandsia comarapaensis (150.2) is related to T. didisticha (150) and therefore may have certain horticultural qualities in common with that species. 3) Genus and species names follow the respective numbers. 4) Subspecific taxa (subspecies, varieties, forms) names are indented below the species names. Note: Variety "a" (the type variety) is not listed unless it contains a form (see Aechmea caudata ). Similarly, the type form is not listed. 5) Author name follows the specific and subspecific names. These names are included for the convenience of specialist users of the list. This list does not contain publication data or synonymy, as it is not our intent for it to be a technical nomenclatural guide. -
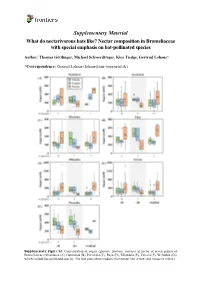
Supplementary Material What Do Nectarivorous Bats Like? Nectar Composition in Bromeliaceae with Special Emphasis on Bat-Pollinated Species
Supplementary Material What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species Author: Thomas Göttlinger, Michael Schwerdtfeger, Kira Tiedge, Gertrud Lohaus* *Correspondence: Gertrud Lohaus ([email protected]) Supplementary Figure S1: Concentration of sugars (glucose, fructose, sucrose) in nectar of seven genera of Bromeliaceae (Alcantarea (A), Guzmania (B), Pitcairnia (C), Puya (D), Tillandsia (E), Vriesea (F), Werauhia (G)) which include bat-pollinated species. The box plots show medians (horizontal line in box) and means (x in box). Supplementary Material What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species Author: Thomas Göttlinger, Michael Schwerdtfeger, Kira Tiedge, Gertrud Lohaus* *Correspondence: Gertrud Lohaus ([email protected]) Supplementary Figure S2: Concentration of amino acids (ala, arg, asn, asp, gaba, gln, glu, gly, his, iso, leu, lys, met, phe, pro, ser, thr, trp, tyr, val) in nectar of seven genera of Bromeliaceae (Alcantarea (A), Guzmania (B), Pitcairnia (C), Puya (D), Tillandsia (E), Vriesea (F), Werauhia (G)), which include bat-pollinated species. The box plots show medians (horizontal line in box) and means (x in box). Supplementary Material What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species Author: Thomas Göttlinger, Michael Schwerdtfeger, Kira Tiedge, Gertrud Lohaus* *Correspondence: Gertrud Lohaus ([email protected]) Supplementary Figure S3: Cation concentrations (Ca2+, K+, Na+, Mg2+) in nectar of seven genera of Bromeliaceae (Alcantarea (A), Guzmania (B), Pitcairnia (C), Puya (D), Tillandsia (E), Vriesea (F), Werauhia (G)), which include bat-pollinated species. The box plots show medians (horizontal line in box) and means (x in box). -

Methanogens Diversity During Anaerobic Sewage Sludge Stabilization and the Effect of Temperature
processes Article Methanogens Diversity during Anaerobic Sewage Sludge Stabilization and the Effect of Temperature Tomáš Vítˇez 1,2, David Novák 3, Jan Lochman 3,* and Monika Vítˇezová 1,* 1 Department of Experimental Biology, Faculty of Science, Masaryk University, 62500 Brno, Czech Republic; [email protected] 2 Department of Agricultural, Food and Environmental Engineering, Faculty of AgriSciences, Mendel University, 61300 Brno, Czech Republic 3 Department of Biochemistry, Faculty of Science, Masaryk University, 62500 Brno, Czech Republic; [email protected] * Correspondence: [email protected] (J.L.); [email protected] (M.V.); Tel.: +420-549-495-602 (J.L.); Tel.: +420-549-497-177 (M.V.) Received: 29 June 2020; Accepted: 10 July 2020; Published: 12 July 2020 Abstract: Anaerobic sludge stabilization is a commonly used technology. Most fermenters are operated at a mesophilic temperature regime. Modern trends in waste management aim to minimize waste generation. One of the strategies can be achieved by anaerobically stabilizing the sludge by raising the temperature. Higher temperatures will allow faster decomposition of organic matter, shortening the retention time, and increasing biogas production. This work is focused on the description of changes in the community of methanogenic microorganisms at different temperatures during the sludge stabilization. At higher temperatures, biogas contained a higher percentage of methane, however, there was an undesirable accumulation of ammonia in the fermenter. Representatives of the hydrogenotrophic genus Methanoliea were described at all temperatures tested. At temperatures up to 50 ◦C, a significant proportion of methanogens were also formed by acetoclastic representatives of Methanosaeta sp. and acetoclastic representatives of the order Methanosarcinales. -

Epilist 1.0: a Global Checklist of Vascular Epiphytes
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2021 EpiList 1.0: a global checklist of vascular epiphytes Zotz, Gerhard ; Weigelt, Patrick ; Kessler, Michael ; Kreft, Holger ; Taylor, Amanda Abstract: Epiphytes make up roughly 10% of all vascular plant species globally and play important functional roles, especially in tropical forests. However, to date, there is no comprehensive list of vas- cular epiphyte species. Here, we present EpiList 1.0, the first global list of vascular epiphytes based on standardized definitions and taxonomy. We include obligate epiphytes, facultative epiphytes, and hemiepiphytes, as the latter share the vulnerable epiphytic stage as juveniles. Based on 978 references, the checklist includes >31,000 species of 79 plant families. Species names were standardized against World Flora Online for seed plants and against the World Ferns database for lycophytes and ferns. In cases of species missing from these databases, we used other databases (mostly World Checklist of Selected Plant Families). For all species, author names and IDs for World Flora Online entries are provided to facilitate the alignment with other plant databases, and to avoid ambiguities. EpiList 1.0 will be a rich source for synthetic studies in ecology, biogeography, and evolutionary biology as it offers, for the first time, a species‐level overview over all currently known vascular epiphytes. At the same time, the list represents work in progress: species descriptions of epiphytic taxa are ongoing and published life form information in floristic inventories and trait and distribution databases is often incomplete and sometimes evenwrong. -

A Case of Communal Egg-Laying of Gonatodes Albogularis (Sauria, Sphaerodactylidae) in Bromeliads (Poales, Bromeliaceae)
Herpetozoa 32: 45–49 (2019) DOI 10.3897/herpetozoa.32.e35663 A case of communal egg-laying of Gonatodes albogularis (Sauria, Sphaerodactylidae) in bromeliads (Poales, Bromeliaceae) Valentina de los Ángeles Carvajal-Ocampo1, María Camila Ángel-Vallejo1, Paul David Alfonso Gutiérrez-Cárdenas2, Fabiola Ospina-Bautista1, Jaime Vicente Estévez Varón1 1 Grupo de Investigación en Ecosistemas Tropicales, Facultad de Ciencias Exactas y Naturales, Universidad de Caldas, Calle 65 # 26-10, A.A 275, Manizales, Colombia 2 Grupo de Ecología y Diversidad de Anfibios y Reptiles, Facultad de Ciencias Exactas y Naturales, Universidad de Caldas, Calle 65 # 26-10, A.A 275, Manizales, Colombia http://zoobank.org/40E4D4A7-C107-46C8-BAB3-01B193722A17 Corresponding author: Valentina de los Ángeles Carvajal-Ocampo ([email protected]) Academic editor: Günter Gollmann ♦ Received 26 September 2018 ♦ Accepted 5 January 2019 ♦ Published 13 May 2019 Abstract The Neotropical Yellow-Headed Gecko Gonatodes albogularis commonly use cavities in the trees as a microhabitat for egg-laying. Here, we present the first record of this species in Colombia using the tank bromeliadTillandsia elongata as nesting sites, along with the occurrence of communal egg-laying in that microhabitat. Key Words Andean disturbed, Colombia, forests, communal egg-laying, nesting sites, Tillandsia elongata Introduction Anadia (Mendoza and Rodríguez-Barbosa 2017), Anolis (Rand 1967; Estrada 1987; Montgomery et al. 2011), Go- Tank bromeliads (Bromeliaceae) are phytotelmata that natodes (Quesnel 1957; Rivero-Blanco 1964; Vitt et al. potentially provide humidity, resources and shelter to ver- 1997; Oda 2004; Rivas Fuenmayor et al. 2006; Jablon- tebrates (Benzing 2000; Schaefer and Duré 2011; Silva ski 2015), Gymnodactylus (Cassimiro and Rodrigues et al. -

The Genus Guzmania (Bromeliaceae) in Venezuela
The genus Guzmania (Bromeliaceae) in Venezuela Compiled by Yuribia Vivas Fundación Instituto Botánico de Venezuela Bruce Holst & Harry Luther Marie Selby Botanical Gardens The genus Guzmania was described by Hipólito Ruiz and José Pavón in 1802 in the "Flora Peruviana et Chilensis." The type species is Guzmania tricolor Ruiz & Pav. The name honors Spanish naturalist Anastasio Guzmán, a student of South American plants and animals (Grant & Zijlstra 1998). Species of Guzmania are distributed from the southern USA (Florida) and Mexico to Brazil and Peru, including the Most species of Guzmania are found in cloud forests at middle elevations. Antilles; they are largely absent from lowland Amazonia. Photograph by Yuribia Vivas. Figure modified from Smith & Downs, Flora Neotropica. Guzmania is placed in the subfamily Tillandsioideae, and is distinguished from other members of the subfamily (Vriesea,Tillandsia, Catopsis, Racinaea, Alcantarea, Mezobromelia, and Werauhia) by having polystichously arranged flowers (that is, arranged in many planes on the inflorescence axis), white, whitish, yellow, or greenish petals that lack nectar scales, and having generally reddish brown-colored seeds. In general aspect, Guzmania is difficult to distinguish from Mezobromelia since both are polystichously flowered and may have similar color schemes, but the presence of nectar scales in Mezobromelia and absence inGuzmania separates them. Approximately 200 species and 17 varieties of Guzmania are known, making it the third largest genus in the subfamily, after Tillandsia and Vriesea. The table below is a listing of Guzmania in Venezuela, with synonymy, types, phenology, and distribution. Column two contains photographs of live plants and the third column, type specimens. Click on the photos for enlarged images. -

Adaptive Radiation, Correlated and Contingent Evolution, and Net Species Diversification in Bromeliaceae
Molecular Phylogenetics and Evolution 71 (2014) 55–78 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae Thomas J. Givnish a,*, Michael H.J. Barfuss b, Benjamin Van Ee c, Ricarda Riina d, Katharina Schulte e,f, Ralf Horres g, Philip A. Gonsiska a, Rachel S. Jabaily h, Darren M. Crayn f, J. Andrew C. Smith i, Klaus Winter j, Gregory K. Brown k, Timothy M. Evans l, Bruce K. Holst m, Harry Luther n, Walter Till b, Georg Zizka e, Paul E. Berry o, Kenneth J. Sytsma a a Department of Botany, University of Wisconsin-Madison, Madison, WI 53706, USA b Department of Systematic and Evolutionary Botany, Faculty of Life Sciences, University of Vienna, Vienna A-1030, Austria c School of Natural Sciences, Black Hills State University, Spearfish, SD 57799, USA d Real Jardín Botánico, CSIC, Plaza de Murillo 2, Madrid 28014, Spain e Department of Botany and Molecular Evolution, Research Institute Senckenberg and J.W. Goethe University, Frankfurt am Main D-60325, Germany f Australian Tropical Herbarium, James Cook University, Cairns, QLD 4878, Australia g GenXPro, Frankfurt am Main 60438, Germany h Department of Biology, Rhodes College, Memphis, TN 38112, USA i Department of Plant Sciences, University of Oxford, Oxford OX1 3RB, United Kingdom j Smithsonian Tropical Research Institute, Balboa, Ancon, Republic of Panama k Department of Botany, University of Wyoming, Laramie, WY 82071, USA l Department of Biology, Grand Valley State University, Allendale, MI 49401, USA m Marie Selby Botanical Gardens, Sarasota, FL 34236, USA n Gardens By The Bay, National Parks Board Headquarters, Singapore 259569, Singapore o Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, MI 48109, USA article info abstract Article history: We present an integrative model predicting associations among epiphytism, the tank habit, entangling Received 22 May 2013 seeds, C3 vs.