What Should Be the Elements of Any Settlement with the Tobacco Industry?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TOBACCO WORLD RETAIL PRICES (Ovor 5,000 Retail PI-ICM)
THE CIGAR AND THE TOBACCO WORLD THE POPULAR JOURNAL TOBACCO OVER 40 YEARS OF TRADE USEFULNESS WORLD The Subscription includes : TOBACCO WORLD RETAIL PRICES (Ovor 5,000 Retail PI-ICM). RETAIL PRICES THE TOBACCO WORLD ANNUAL (Containing a word of Trad* Brand*—with Nam* and Addrau In each cms*). Membership of: TOBACCO WORLD SERVICE JUNE 1935 (With Pott Fnta raplUa In all Trad* difficult!**). The Cigar & Tobacco World HIYWOOO A COMPANY LTD. Dmrr How*, Kin—U 3tr*M, Ontry Una, London, W.C1 trantfc OACM f Baadmur. •trmlnfhtn, Uteanar. ToWfTHM i OffUlfrunt, Phono, LonAon. •Phono I TomaU far M1J Published by THE CIGAR & TOBACCO WORLD HEYWOOD & CO., LTD. DRURY HOUSE, RUSSELL STREET, DRURY LANE, LONDON, W.C.2 Branch Offices: MANCHESTER, BIRMINGHAM, LEICESTER Talagrarm : "Organigram. Phono, London." Phono : Tampla Bar MZJ '' Inar) "TOBACCO WORLD" RETAIL PRICES 1935 Authorised retail prices of Tobaccos, Cigarettes, Fancy Goods, and Tobacconists' Sundries. ABDULLA & Co., Ltd. (\BDULD^ 173 New Bond Street, W.l. Telephone; Bishopsgnte 4815, Authorised Current Retail Prices. Turkish Cigarettes. Price per Box of 100 50 25 20 10 No. 5 14/6 7/4 3/8 — 1/6 No. 5 .. .. Rose Tipped .. 28/9 14/6 7/3 — 3/- No. 11 11/8 5/11 3/- - 1/3 No. II .. .. Gold Tipped .. 13/S 6/9 3/5 - No. 21 10/8 5/5 2/9 — 1/1 Turkish Coronet No. 1 7/6 3/9 1/10J 1/6 9d. No. "X" — 3/- 1/6 — — '.i^Sr*** •* "~)" "Salisbury" — 2/6 — 1/- 6d. Egyptian Cigarettes. No. 14 Special 12/5 6/3 3/2 — — No. -

Appendix 1. Categorization of Cigarette Brands As Either Premium Or Discount
Appendix 1. Categorization of Cigarette Brands as either Premium or Discount Category Name of Cigarette Brand Premium Accord, American Spirit, Barclay, Belair, Benson & Hedges, Camel, Capri, Carlton, Chesterfield, Davidoff, Du Maurier, Dunhill, Dunhill International, Eve, Kent, Kool, L&M, Lark, Lucky Strike, Marlboro, Max, Merit, Mild Seven, More, Nat Sherman, Newport, Now, Parliament, Players, Quest, Rothman’s, Salem, Sampoerna, Saratoga, Tareyton, True, Vantage, Virginia Slims, Winston, Raleigh, Business Club Full Flavor, Ronhill, Dreams Discount 24/7, 305, 1839, A1, Ace, Allstar, Allway Save, Alpine, American, American Diamond, American Hero, American Liberty, Arrow, Austin, Axis, Baileys, Bargain Buy, Baron, Basic, Beacon, Berkeley, Best Value, Black Hawk, Bonus Value, Boston, Bracar, Brand X, Brave, Brentwood, Bridgeport, Bronco, Bronson, Bucks, Buffalo, BV, Calon, Cambridge, Campton, Cannon, Cardinal, Carnival, Cavalier, Champion, Charter, Checkers, Cherokee, Cheyenne, Cimarron, Circle Z, Class A, Classic, Cobra, Complete, Corona, Courier, CT, Decade, Desert Gold, Desert Sun, Discount, Doral, Double Diamond, DTC, Durant, Eagle, Echo, Edgefield, Epic, Esquire, Euro, Exact, Exeter, First Choice, First Class, Focus, Fortuna, Galaxy Pro, Gauloises, Generals, Generic/Private Label, Geronimo, Gold Coast, Gold Crest, Golden Bay, Golden, Golden Beach, Golden Palace, GP, GPC, Grand, Grand Prix, G Smoke, GT Ones, Hava Club, HB, Heron, Highway, Hi-Val, Jacks, Jade, Kentucky Best, King Mountain, Kingsley, Kingston, Kingsport, Knife, Knights, -

North Dakota Office of State Tax Commissioner Tobacco Directory List of Participating Manufacturers (Listing by Brand) As of May 24, 2019
North Dakota Office of State Tax Commissioner Tobacco Directory List of Participating Manufacturers (Listing by Brand) As of May 24, 2019 **RYO: Roll-Your-Own Brand Name Manufacturer 1839 U.S. Flue-Cured Tobacco Growers, Inc. 1839 RYO U.S. Flue-Cured Tobacco Growers, Inc. 1st Class U.S. Flue-Cured Tobacco Growers, Inc. American Bison RYO Wind River Tobacco Company, LLC Amsterdam Shag RYO Peter Stokkebye Tobaksfabrik A/S Ashford RYO Von Eicken Group Bali Shag RYO Commonwealth Brands, Inc. Baron American Blend Farmer’s Tobacco Co of Cynthiana, Inc. Basic Philip Morris USA, Inc. Benson & Hedges Philip Morris USA, Inc. Black & Gold Sherman’s 1400 Broadway NYC, LLC Bo Browning RYO Top Tobacco, LP Bugler RYO Scandinavian Tobacco Group Lane Limited Bull Brand RYO Von Eicken Group Cambridge Philip Morris USA, Inc. Camel R.J. Reynolds Tobacco Company Camel Wides R.J. Reynolds Tobacco Company Canoe RYO Wind River Tobacco Company, LLC Capri R.J. Reynolds Tobacco Company Carlton R.J. Reynolds Tobacco Company CF Straight Virginia RYO Von Eicken Group Charles Fairmon RYO Von Eicken Group Chesterfield Philip Morris USA, Inc. Chunghwa Konci G & D Management Group (USA) Inc. Cigarettellos Sherman’s 1400 Broadway NYC, LLC Classic Sherman’s 1400 Broadway NYC, LLC Classic Canadian RYO Top Tobacco, LP Commander Philip Morris USA, Inc. Crowns Commonwealth Brands Inc. Custom Blends RYO Wind River Tobacco Company, LLC Brand Name Manufacturer Danish Export RYO Peter Stokkebye Tobaksfabrik A/S Dark Fired Shag RYO Von Eicken Group Dave’s Philip Morris USA, Inc. Davidoff Commonwealth Brands, Inc. Djarum P.T. -

Parking Lot Sobriety· Test Threatened Inside the Deer Park Parking Lot, Which Runs Directly Behind Known for Its Large and the Tavern
Bulk Rate U.S. Postage PAID iPermit No.320 Newark, DE. University of Delaware, Newark, Delaware Friday, May 4, 1979 UD Union Members Hold Demonstration · Workers Protest Contract Procedures By LISA BARTH descriptions, inadequate ar bitration and discrepencies The university's unionized on vacation and sick leaves hourly-paid employees are the major contractual demonstrated outside disputes, said Evans. Hullihen Hall Wednesday to Over 300 workers from voice complaints concerning maintenance, food service, their contract. plant operation,s and housing protested at various periods throughout the day. Most at tended during their lunch hours. More than 500 university employees are members of the American Federation of State, County and Municipal Employees' Local439. Evans said the demonstration was a "test" to see how many of the members would . support the Review photo by Neal Williamson union fight. 300 UNION WORKERS DEMONSTRATED for more voice in contract procedures near Hullihen However, the demonstra- Hall Wednesday. tion was obscured by the Union members specifical- differ in pay scale. "There weeks beyond the set limit, he "May Day On the Mall" ac- ly complained that the ad are cooks doing janitorial added. tivities held nearby. ministration last summer work and being paid higher "I really felt bad for them. hired two carpenters without wages than the janitors," he ~vans said he sent the They went completely un- first posting the job openings said. "There are even cooks grievances through campus noticed, said Harold Brown, for union-member bids, as cleaning bathrooms who then mail within the specified time UNION PRESIDENT BOB vice president of administra- stated in the contract, Evans go back and handle food.'' limit. -

Cigarette Minimum Retail Price List
MASSACHUSETTS DEPARTMENT OF REVENUE FILING ENFORCEMENT BUREAU CIGARETTE AND TOBACCO EXCISE UNIT PRESUMPTIVE MINIMUM RETAIL PRICES EFFECTIVE July 26, 2021 The prices listed below are based on cigarettes delivered by the wholesaler and do not include the 6.25 percent sales tax. Brands of cigarettes held in current inventory may be sold at the new presumptive minimum prices for those brands. Changes and additions are bolded. Non-Chain Stores Chain Stores Retail Retail Brand (Alpha) Carton Pack Carton Pack 1839 $86.64 $8.66 $85.38 $8.54 1st Class $71.49 $7.15 $70.44 $7.04 Basic $122.21 $12.22 $120.41 $12.04 Benson & Hedges $136.55 $13.66 $134.54 $13.45 Benson & Hedges Green $115.28 $11.53 $113.59 $11.36 Benson & Hedges King (princess pk) $134.75 $13.48 $132.78 $13.28 Cambridge $124.78 $12.48 $122.94 $12.29 Camel All others $116.56 $11.66 $114.85 $11.49 Camel Regular - Non Filter $141.43 $14.14 $139.35 $13.94 Camel Turkish Blends $110.14 $11.01 $108.51 $10.85 Capri $141.43 $14.14 $139.35 $13.94 Carlton $141.43 $14.14 $139.35 $13.94 Checkers $71.54 $7.15 $70.49 $7.05 Chesterfield $96.53 $9.65 $95.10 $9.51 Commander $117.28 $11.73 $115.55 $11.56 Couture $72.23 $7.22 $71.16 $7.12 Crown $70.76 $7.08 $69.73 $6.97 Dave's $107.70 $10.77 $106.11 $10.61 Doral $127.10 $12.71 $125.23 $12.52 Dunhill $141.43 $14.14 $139.35 $13.94 Eagle 20's $88.31 $8.83 $87.01 $8.70 Eclipse $137.16 $13.72 $135.15 $13.52 Edgefield $73.41 $7.34 $72.34 $7.23 English Ovals $125.44 $12.54 $123.59 $12.36 Eve $109.30 $10.93 $107.70 $10.77 Export A $120.88 $12.09 $119.10 $11.91 -

Release of Carbon Granules from Cigarettes with Charcoal Filters
1997;6:33-4Q 33 Tob Control: first published as 10.1136/tc.6.1.33 on 1 March 1997. Downloaded from Release of carbon granules from cigarettes with charcoal filters John L Pauly, Sharon J Stegmeier, Andrew G Mayer, Joel D Lesses, Richard J Streck Abstract Keywords: cigarette filter; charcoal; fibres; gas Objective~~-To inspect cigarettes with a Most (more than 95%) of cigarettes marketed triple granular filter for charcoal granules 1 5 on the cut filter surface and, if present, to today in the United States have filters. " We determine whether the charcoal granules believe that the smoker perceives the filter of a on the filter are released during smoking. cigarette to be both safe and efficient. Design—400 Lark cigarettes in 20 packs However, recent observations in our laboratory were examined individually by each of challenged this view. For example, we have observed the release of cellulose acetate fibres three investigators for the presence of 1 charcoal granules on the cut surface of the from cigarette filters.' These filter fibres were: cellulose acetate filter. Without removing (a) observed trapped between the cellophane the cigarettes from the pack, the filters wrapper and the unopened pack of cigarettes; were examined with a stereo zoom micro- (b) present in the residue at the bottom of scope for charcoal granules. The percent- packs; (c) discharged from the filter when ciga- age of cigarettes that had charcoal rettes were tapped from a height of 3.5 cm or granules was defined, and charcoal dropped from 15 cm; (d) liberated when the granules on each filter were counted. -

October 2019 Author Southeast Asia Tobacco Control Alliance (SEATCA)
October 2019 Author Southeast Asia Tobacco Control Alliance (SEATCA) Cover Design and Layout Wendell Balderas Acknowledgements This regional report is based on a review of intellectual property registration of tobacco brands in five ASEAN countries: Indonesia, Malaysia, Philippines, Thailand, and Vietnam. SEATCA would like to thank the following country collaborators and researchers: Indonesia: Mr. Tubagus Haryo Karbyanto, Jakarta Resident’s Forum Malaysia: Mr. Yong Check Yoon, Consultant Philippines: Mr. Allan Chester Nadate, Consultant Thailand: Dr. Roengrudee Patanavanich, Department of Community Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University Vietnam: Dr. Nguyen Thi Thu Hien, Department of Economics, Thuongmai University Suggested Citation Southeast Asia Tobacco Control Alliance. Big Tobacco’s smoke-free deception: Tobacco trademarks in ASEAN countries uncover the truth. October 2019, Southeast Asia Tobacco Control Alliance (SEATCA), Bangkok, Thailand. The information, findings, interpretations, and conclusions expressed herein are those of the author(s) and do not necessarily reflect the views of the funding organization, its staff, or its Board of Directors. While reasonable efforts have been made to ensure the accuracy of the information presented at the time of publication, SEATCA does not guarantee the completeness and accuracy of the information in this document and shall not be liable for any damages incurred as a result of its use. Any factual errors or omissions are unintentional. For any corrections, please contact SEATCA at [email protected]. © Southeast Asia Tobacco Control Alliance 2019 This document is the intellectual property of SEATCA and its authors. SEATCA retains copyright on all text and graphics in this document, unless indicated otherwise. This copyright is protected by domestic copyright laws and international treaty provisions. -

Application Note VITROCELL® Holder Systems for E-Cigarettes – Secure
AN 005 -07/20 Application Note VITROCELL® holder systems for e-cigarettes – secure and tight connection of any device to the smoking machine New designs of electronic cigarettes can be used. The such as ENDS (Electronic Nicotine compati bility is Delivery Systems) products or HTP tested with JUUL, (Heated Tobacco Products) lead to a myblu, Alto, Logic, large variety of different shapes which IQOS, vype, GLO make the insertion into conventional and tank products. holders with labyrinth seals impossible. VITROCELL® holders are tight, very solidly worked out, durable and easy to clean. They are compatible with all VITROCELL® Secure and tight connection Smoking Machines & Robots. Vitrocell VC 1 S-Type of any device. smoking machine with In most cases the exchange Vapestarter Rack for VITROCELL® has developed a new holder of the inner sealing is sufficient different e-cigarette system which is flexible to adjust to to adjust for a specific shape. devices. different shapes. Thus, all e-cigarettes VITROCELL® Vapestarter – For an automatic button activation of e-cigarettes Button activated e-cigarettes put VITROCELL® Vapestarter is the automated smoking machine users into a problem: solution to press the button in a precise Features: should one press the button every 30 manner . You can connect the device to ○ Integration into software of or 60 seconds manually? Also, Heated any VITROCELL® smoking machine. VC 1, VC 1 S-TYPE, VC 1/7, VC 10® Tobacco Products need to be activated The trigger function is controlled by the and VC 10® S-TYPE Smoking prior to the first puff. software of the smoke generator. -

Case No COMP/M.3191 - PHILIP MORRIS / PAPASTRATOS
EN Case No COMP/M.3191 - PHILIP MORRIS / PAPASTRATOS Only the English text is available and authentic. REGULATION (EEC) No 4064/89 MERGER PROCEDURE Article 6(1)(b) NON-OPPOSITION Date: 02/10/2003 Also available in the CELEX database Document No 303M3191 Office for Official Publications of the European Communities L-2985 Luxembourg COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 02/10/2003 SG (2003) D/232408 In the published version of this decision, PUBLIC VERSION some information has been omitted pursuant to Article 17(2) of Council Regulation (EEC) No 4064/89 concerning non-disclosure of MERGER PROCEDURE business secrets and other confidential ARTICLE 6(1)(b) DECISION information. The omissions are shown thus […]. Where possible the information omitted has been replaced by ranges of figures or a To the notifying parties general description. Dear Sir/Madam, Subject: Case No COMP/M.3191 - PHILIP MORRIS/PAPASTRATOS Notification of 2.9.2003 pursuant to Article 4 of Council Regulation No 4064/891 1. On 2.9.2003, Philip Morris Holland BV notified its intention to acquire control of the whole of Papastratos Cigarette Manufacturing SA (“Papastratos”) within the meaning of Art 3(1)b of the Merger Regulation. 2. The Commission has concluded that the notified operation falls within the scope of the Merger Regulation as amended and does not raise serious doubts as to its compatibility with the common market and with the functioning of the EEA Agreement. I. THE PARTIES 3. Philip Morris Holland BV is a subsidiary of Philip Morris International Inc. ("Philip Morris"), an affiliate of Altria Group, Inc. -
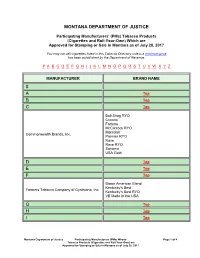
Pms) Whose Tobacco Products (Cigarettes and Roll-Your-Own
MONTANA DEPARTMENT OF JUSTICE Participating Manufacturers' (PMs) Tobacco Products (Cigarettes and Roll-Your-Own) Which are Approved for Stamping or Sale in Montana as of July 28, 2017 You may not sell cigarettes listed in this Tobacco Directory unless a minimum price has been established by the Department of Revenue. # A B C D E F G H I J K L M N O P Q R S T U V W X Y Z MANUFACTURER BRAND NAME # A Top B Top C Top Bali Shag RYO Crowns Fortuna McClintock RYO Montclair Commonwealth Brands, Inc. Premier RYO Rave Rave RYO Sonoma USA Gold D Top E Top F Top Baron American Blend Kentucky's Best Farmers Tobacco Company of Cynthiana, Inc. Kentucky's Best RYO VB Made in the USA G Top H Top I Top Montana Department of Justice Participating Manufacturers (PMs) Whose Page 1 of 4 Tobacco Products (Cigarettes and Roll-Your-Own) are Approved for Stamping or Sale in Montana as of July 28, 2017 MANUFACTURER BRAND NAME Kool Maverick ITG Brands, LLC Salem Winston J Top Export A Japan Tobacco International U.S.A., Inc. LD by L. Ducat Wave K Top Ace Checkers King Maker Marketing, Inc. Gold Crest Hi-Val Konci G&D Management Group (USA), Inc. Golden Deer L Top Eve Grand Prix Liggett Group, LLC Liggett Select Pyramid M Top N Top Red Sun NASCO Products, LLC Smoker Friendly (SF) O Top P Top P.S. RYO: Amsterdam Shag RYO Danish Export RYO Peter Stokkebye Tobaksfabrik A/S London Export RYO Norwegian Shag RYO Stockholm Blend RYO Turkish Export RYO Montana Department of Justice Participating Manufacturers (PMs) Whose Page 2 of 4 Tobacco Products (Cigarettes and Roll-Your-Own) are Approved for Stamping or Sale in Montana as of July 28, 2017 MANUFACTURER BRAND NAME Basic Benson & Hedges Cambridge Chesterfield Commander Dave's English Ovals Philip Morris USA, Inc. -

Page 1 of 15
Updated September14, 2021– 9:00 p.m. Date of Next Known Updates/Changes: *Please print this page for your own records* If there are any questions regarding pricing of brands or brands not listed, contact Heather Lynch at (317) 691-4826 or [email protected]. EMAIL is preferred. For a list of licensed wholesalers to purchase cigarettes and other tobacco products from - click here. For information on which brands can be legally sold in Indiana and those that are, or are about to be delisted - click here. *** PLEASE sign up for GovDelivery with your EMAIL and subscribe to “Tobacco Industry” (as well as any other topic you are interested in) Future lists will be pushed to you every time it is updated. *** https://public.govdelivery.com/accounts/INATC/subscriber/new RECENTLY Changed / Updated: 09/14/2021- Changes to LD Club and Tobaccoville 09/07/2021- Update to some ITG list prices and buydowns; Correction to Pall Mall buydown 09/02/2021- Change to Nasco SF pricing 08/30/2021- Changes to all Marlboro and some RJ pricing 08/18/2021- Change to Marlboro Temp. Buydown pricing 08/17/2021- PM List Price Increase and Temp buydown on all Marlboro 01/26/2021- PLEASE SUBSCRIBE TO GOVDELIVERY EMAIL LIST TO RECEIVE UPDATED PRICING SHEET 6/26/2020- ***RETAILER UNDER 21 TOBACCO***(EFF. JULY 1) (on last page after delisting) Minimum Minimum Date of Wholesale Wholesale Cigarette Retail Retail Brand List Manufacturer Website Price NOT Price Brand Price Per Price Per Update Delivered Delivered Carton Pack Premier Mfg. / U.S. 1839 Flare-Cured Tobacco 7/15/2021 $42.76 $4.28 $44.00 $44.21 Growers Premier Mfg. -

38 2000 Tobacco Industry Projects—A Listing (173 Pp.) Project “A”: American Tobacco Co. Plan from 1959 to Enlist Professor
38 2000 Tobacco Industry Projects—a Listing (173 pp.) Project “A”: American Tobacco Co. plan from 1959 to enlist Professors Hirsch and Shapiro of NYU’s Institute of Mathematical Science to evaluate “statistical material purporting to show association between smoking and lung cancer.” Hirsch and Shapiro concluded that “such analysis is not feasible because the studies did not employ the methods of mathematical science but represent merely a collection of random data, or counting noses as it were.” Statistical studies of the lung cancer- smoking relation were “utterly meaningless from the mathematical point of view” and that it was “impossible to proceed with a mathematical analysis of the proposition that cigarette smoking is a cause of lung cancer.” AT management concluded that this result was “not surprising” given the “utter paucity of any direct evidence linking smoking with lung canner.”112 Project A: Tobacco Institute plan from 1967 to air three television spots on smoking & health. Continued goal of the Institute to test its ability “to alter public opinion and knowledge of the asserted health hazards of cigarette smoking by using paid print media space.” CEOs in the fall of 1967 had approved the plan, which was supposed to involve “before-and-after opinion surveys on elements of the smoking and health controversy” to measure the impact of TI propaganda on this issue.”113 Spots were apparently refused by the networks in 1970, so plan shifted to Project B. Project A-040: Brown and Williamson effort from 1972 to 114 Project AA: Secret RJR effort from 1982-84 to find out how to improve “the RJR share of market among young adult women.” Appeal would 112 Janet C.