Effect of Water Velocity and Temperature On
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Iucn Red Data List Information on Species Listed On, and Covered by Cms Appendices
UNEP/CMS/ScC-SC4/Doc.8/Rev.1/Annex 1 ANNEX 1 IUCN RED DATA LIST INFORMATION ON SPECIES LISTED ON, AND COVERED BY CMS APPENDICES Content General Information ................................................................................................................................................................................................................................ 2 Species in Appendix I ............................................................................................................................................................................................................................... 3 Mammalia ............................................................................................................................................................................................................................................ 4 Aves ...................................................................................................................................................................................................................................................... 7 Reptilia ............................................................................................................................................................................................................................................... 12 Pisces ................................................................................................................................................................................................................................................. -

Characterization of Pallid Sturgeon (<I>Scaphirhynchus Albus</I
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey 2020 Characterization of Pallid Sturgeon (Scaphirhynchus albus) Spawning Habitat in the Lower Missouri River Caroline M. Elliott Aaron J. DeLonay Kimberly Chojnacki Robert B. Jacobson Follow this and additional works at: https://digitalcommons.unl.edu/usgsstaffpub Part of the Geology Commons, Oceanography and Atmospheric Sciences and Meteorology Commons, Other Earth Sciences Commons, and the Other Environmental Sciences Commons This Article is brought to you for free and open access by the US Geological Survey at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Staff -- Published Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Received: 3 June 2019 | Revised: 27 November 2019 | Accepted: 3 December 2019 DOI: 10.1111/jai.13994 STURGEON PAPER Characterization of Pallid Sturgeon (Scaphirhynchus albus) Spawning Habitat in the Lower Missouri River Caroline M. Elliott | Aaron J. DeLonay | Kimberly A. Chojnacki | Robert B. Jacobson Columbia Environmental Research Center, U.S. Geological Survey, Columbia, MO, USA Abstract Acipenseriformes (sturgeons and paddlefish) globally have declined throughout their Correspondence Caroline M. Elliott, Columbia Environmental range due to river fragmentation, habitat loss, overfishing, and degradation of water Research Center, U.S. Geological Survey, quality. In North America, pallid sturgeon (Scaphirhynchus albus) populations have ex- 4200 New Haven Rd, Columbia, MO 65201, USA. perienced poor to no recruitment, or substantial levels of hybridization with the closely Email: [email protected] related shovelnose sturgeon (S. platorynchus). The Lower Missouri River is the only por- Funding information tion of the species’ range where successful reproduction and recruitment of genetically U. -

Fisheries Research Report 1936 December 9, 1985
1 9 3 6 A Partial Bibliography for the Sturgeon Family Acipenseridae · Eric R. Anderson Fisheries Research Report No. 1936 December 9, 1985 MICHIGAN DEPARTMENT OF NATURAL RESOURCES FISHERIES DIVISION Fisheries Research Report 1936 December 9, 1985 A PARTIAL BIBLIOGRAPHY FOR THE STURGEON FAMILY ACIPENSERIDAE Eric R. Anderson 2 INTRODUCTION Sturgeon are large, primitive fishes that mature late, are long-lived, and occur in both freshwater and marine systems throughout the world. The bibliography presented here consists of 288 references divided into systematics and distribution, biology, management, morphology, physiology, and fish health for 23 species of the genera Acipenser, Huso, Pseudoscaphirhynchus, and Scaphirhvnchus. In the biology section, references are further subdivided into general biology, reproduction, feeding and locomotion, early life history, and age and growth. In the management section, references are subdivided into fishery history, population dynamics, and cultural practices. Within each section references are arranged in alphabetical order by the author's surname. 3 SYSTEMATICS AND DISTRIBUTION Bailey, R. M., and F. B. Cross. 1954. River sturgeons of the American genus Scaphirhynchus: characters, distribution, and synonymy. Paper of the Michigan Academy of Science, Arts, and Letters 39:169-208. Bajkov, A. D. 1955. White sturgeon with seven rows of scutes. California Fish and Game 41:347-348. Cooper, E. L. 1957. What kind of sturgeon is it? Wisconsin Conservation Bulletin 22:31. Eddy, S. 1945. Paddlefish and sturgeon. Geological relics among Minnesota fishes. The Conservation Volunteer 8:29-32. Filippov, G. M. 1976. Some data on the biology of the beluga Huso huso from the south eastern part of the Caspian Sea. -

Amur Fish: Wealth and Crisis
Amur Fish: Wealth and Crisis ББК 28.693.32 Н 74 Amur Fish: Wealth and Crisis ISBN 5-98137-006-8 Authors: German Novomodny, Petr Sharov, Sergei Zolotukhin Translators: Sibyl Diver, Petr Sharov Editors: Xanthippe Augerot, Dave Martin, Petr Sharov Maps: Petr Sharov Photographs: German Novomodny, Sergei Zolotukhin Cover photographs: Petr Sharov, Igor Uchuev Design: Aleksey Ognev, Vladislav Sereda Reviewed by: Nikolai Romanov, Anatoly Semenchenko Published in 2004 by WWF RFE, Vladivostok, Russia Printed by: Publishing house Apelsin Co. Ltd. Any full or partial reproduction of this publication must include the title and give credit to the above-mentioned publisher as the copyright holder. No photographs from this publication may be reproduced without prior authorization from WWF Russia or authors of the photographs. © WWF, 2004 All rights reserved Distributed for free, no selling allowed Contents Introduction....................................................................................................................................... 5 Amur Fish Diversity and Research History ............................................................................. 6 Species Listed In Red Data Book of Russia ......................................................................... 13 Yellowcheek ................................................................................................................................... 13 Black Carp (Amur) ...................................................................................................................... -

Kaluga Sturgeon, Kaluga Finnish: Amurinkitasampi French: German: Japanese: Dauria-Chôzame Russian: Kaluga Spanish
221 Proposal II / 26 PROPOSAL FOR INCLUSION OF SPECIES ON THE APPENDICES OF THE CONVENTION ON THE CONSERVATION OF MIGRATORY SPECIES OF WILD ANIMALS PROPOSAL: Inclusion of the following species of Huso dauricus in Appendix II of the Convention on the Conservation of Migratory Species of Wild Animals (CMS): B. PROPONENT: Federal Republic of Germany C. SUPPORTING STATEMENT 1. Taxon 1.1_ Classis: Actinopterygii 1.2 Ordo: Acipenseriformes 1.3 Familia: Acipenseridae 1.4 Species: Huso dauricus (Georgi, 1775) 1.5 Common names English: Kaluga sturgeon, Kaluga Finnish: Amurinkitasampi French: German: Japanese: dauria-chôzame Russian: Kaluga Spanish: Name of caviar: Kaluga, Keluga, Amur sturgeon caviar 2. Biological data 2.1 Distribution Huso dauricus is endemic to the Amur River system where it occurs from the delta to the upper reaches including the large tributaries and lakes (Berg, 1948; Nikol´skii,1956). Young individuals are reported to enter the Sea of Okhotsk and the Sea of Japan during the summer months reaching the north-eastern part of the Sakhalin Island, the northern part of the Tatar Strait, the coastal waters of Hokkaido Island and Honshu Island off Niigata (Kostarev and Tyurnin, 1970; Gritsenko and Kostyunin, 1979; Amaoka and Nakaya, 1975; Honma and Itano, 1994). The distribution range of the kaluga in the Amur is fragmented: a population living in the estuary and coastal zones can be distinguished from local populations in the lower Amur, middle Amur and Zeya-Bureya river system (Svirskii, 1971; Krykhtin and Svirskii, 1997a and 1997b). 2.2 Population 222 Krykhtin and Svirskii (1997a and 1997b) give an estimate of the size of the different populations using data of mass marking carried out at the end of the 1980s and calculated data (area method) based on irregular catches in the lower and middle Amur: the estuary population is relatively most abundant and contained almost 70,000 individuals older than one year at the end of the 1980s, of which approximately 5,000 (14%) with a weight exceeding 100 kg were potentially sexually mature. -

Universal Caviar Labelling Requirements
UNIVERSAL CAVIAR LABELLING REQUIREMENTS All sturgeon caviar BACKGROUND containers in domestic and international trade have to All species of sturgeon and paddlefish have been listed in the Appendices of the bear a non-reusable label Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) since 1998. Two species—the Common or Baltic Sturgeon Acipenser sturio containing details about the and the Shortnose Sturgeon Acipenser brevirostrum—are listed in Appendix I of the source and the country of Convention, which prohibits any international commercial trade. All other species origin of the caviar. are listed in Appendix II, whereby international trade is regulated by governments Governments around the through a system of permits. world have agreed to a universal caviar labelling With the aim of facilitating the legal caviar trade around the world and allowing the system with the aim of easy identification of the source and origin of caviar, governments agreed at a CITES ensuring that all caviar meeting in 2000 to introduce a standardized labelling system for all caviar exports. Two years later, they extended the labelling requirements and agreed that all caviar entering the market is from containers in trade, whether imported, exported, re-exported or in domestic legal sources. The caviar markets, should bear a label that would contain a specific set of information, including labelling system helps the country of origin and the year of harvest, to allow identification of the source of governments, traders and the caviar. Governments agreed that, as of January 2004, they would only accept consumers in distinguishing imported caviar shipments marked according to the labelling guidelines. -

And American Paddlefish
G C A T T A C G G C A T genes Article Hybridization of Russian Sturgeon (Acipenser gueldenstaedtii, Brandt and Ratzeberg, 1833) and American Paddlefish (Polyodon spathula, Walbaum 1792) and Evaluation of Their Progeny 1, , 1, 1 1,2 Jen˝oKáldy * y, Attila Mozsár y, Gyöngyvér Fazekas ,Móni Farkas , Dorottya Lilla Fazekas 1,3, Georgina Lea Fazekas 1,4, Katalin Goda 5,6 , Zsuzsanna Gyöngy 5,6, Balázs Kovács 7, Kenneth Semmens 8 , Miklós Bercsényi 2,9, Mariann Molnár 4,10 and Eszter Patakiné Várkonyi 10 1 National Agricultural Research and Innovation Centre, Research Institute for Fisheries and Aquaculture, H-5540 Szarvas, Hungary; [email protected] (A.M.); [email protected] (G.F.); [email protected] (M.F.); [email protected] (D.L.F.); [email protected] (G.L.F.) 2 Festetics György Doctoral School, Georgikon Faculty, University of Pannonia, H-8360 Keszthely, Hungary; [email protected] 3 Juhász-Nagy Pál Doctoral School of Biology and Environmental Sciences, University of Debrecen, H-4031 Debrecen, Hungary 4 Animal Husbandry Science Doctoral School, Szent István University, H-2100 Gödöll˝o,Hungary; [email protected] 5 Department of Biophysics and Cell Biology, Faculty of Medicine, University of Debrecen, H-4032 Debrecen, Hungary; [email protected] (K.G.); [email protected] (Z.G.) 6 Doctoral School of Molecular Cell and Immune Biology, University of Debrecen, H-4032 Debrecen, Hungary 7 Department of Aquaculture, Institute for Conservation of Natural Resources, -

Drift Dynamics of Larval Pallid Sturgeon and Shovelnose Sturgeon in a Natural Side Channel of the Upper Missouri River, Montana
North American Journal of Fisheries Management 28:808–826, 2008 [Article] Ó Copyright by the American Fisheries Society 2008 DOI: 10.1577/M06-285.1 Drift Dynamics of Larval Pallid Sturgeon and Shovelnose Sturgeon in a Natural Side Channel of the Upper Missouri River, Montana PATRICK J. BRAATEN* U.S. Geological Survey, Columbia Environmental Research Center, Fort Peck Project Office, Fort Peck, Montana 59223, USA DAVID B. FULLER,LANDON D. HOLTE,RYAN D. LOTT, AND WILLIAM VISTE Montana Department of Fish, Wildlife and Parks, Fort Peck Fisheries Office, Fort Peck, Montana 59223, USA TYREL F. BRANDT AND ROBERT G. LEGARE U.S. Geological Survey, Fort Peck Field Office, Fort Peck, Montana 59223, USA Abstract.—The drift dynamics of larval shovelnose sturgeon Scaphirhynchus platorynchus (1, 2, 6, and 10 d posthatch [dph]) and pallid sturgeon S. albus (1, 2, 5, 9, 11, and 17 dph) were examined in a natural side channel of the Missouri River to quantify the vertical drift location of larvae in the water column, determine the drift velocity of larvae relative to water velocity, and simulate the cumulative distance (km) drifted by larvae during ontogenetic development. Larvae were released at the side-channel inlet and sampled at points 100, 500, 900, and 1,300 m downstream. Larvae drifted primarily near the riverbed, as 58–79% of recaptured shovelnose sturgeon and 63–89% of recaptured pallid sturgeon were sampled in the lower 0.5 m of the water column. The transition from the drifting to the benthic life stage was initiated at 6 dph (mean length, 15.6 mm) for shovelnose sturgeon and at 11–17 dph (mean length, 18.1–20.3 mm) for pallid sturgeon. -

Status, Trends and Management of Sturgeon and Paddlefish Fisheries
F I S H and F I S H E R I E S , 2005, 6, 233–265 Status, trends and management of sturgeon and paddlefish fisheries Ellen K Pikitch1,2*, Phaedra Doukakis1,2*, Liz Lauck3, Prosanta Chakrabarty4 & Daniel L Erickson3 1Pew Institute for Ocean Science, 126 East 56th Street, Mezzanine, New York, NY 10022; 2University of Miami, Rosenstiel School of Marine and Atmospheric Science, 4600 Rickenbacker Causeway, Key Biscayne, FL 33149–1098; 3Marine Conservation Program, Wildlife Conservation Society, 2300 Southern Blvd., Bronx, NY 10460; 4Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, MI 48109, USA Abstract Correspondence: The 27 extant species of sturgeons and paddlefishes (Order Acipenseriformes) Phaedra Doukakis, Pew Institute for represent a unique and relict lineage of fishes. Producers of coveted black caviar, Ocean Science, 126 sturgeons are one of the most valuable wildlife commodities on earth. The group is East 56th Street, among the most endangered fishes with all species listed under Convention on Mezzanine, New York, International Trade in Endangered Species (CITES) Appendix I (two species) or II (25 NY 10022, USA species), only two species considered Lower Risk by IUCN, four of the nine US taxa Tel.: 212 756 0042 Fax: 212 756 0045 and one Caspian species protected under the Endangered Species Act, and local E-mail: pdoukakis@ extinctions recorded for 19 of 27 species. Despite their well-publicized imperilled rsmas.miami.edu status, commercial pressure on 15 species persists. Here, after reviewing the biological characteristics of sturgeons and paddlefishes and their commercial use, an *These authors overview of global fisheries is presented. -

Sturgeon Hatchery Manual
FAO ISSN 2070-7010 FISHERIES AND 558 AQUACULTURE TECHNICAL PAPER 558 STURGEON HATCHERY MANUAL This Sturgeon Hatchery Manual includes the latest available scientific research findings and experiences and compiles advice given in earlier manuals and handbooks on sturgeon culture and reproduction practices. This document was prepared in response HATC STURGEON to numerous requests for practical guidance on this subject from the Central Asian and Caucasus region to the Food and Agriculture Organization of the United Nations (FAO). This manual is targeted particularly at sturgeon farmers, sturgeon hatchery operators, hatchery technicians, and fisheries and aquaculture managers involved in sturgeon aquaculture development and the restocking and rehabilitation of sturgeon populations in the countries around the basins of the Black and Caspian seas. It aims to provide a practical handbook of modern sturgeon hatchery practices and management. The H manual is available in the English, Russian and Turkish languages. MANUAL ERY FAO FAO Sturgeon Hatchery FISHERIES AND Manual AQUACULTURE TECHNICAL PAPER 558 Prepared by Mikhail S. Chebanov FAO Consultant Krasnodar, Russia and Elena V. Galich Krasnodar, Russia FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Ankara, 2013 Reprinted 2013 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. -
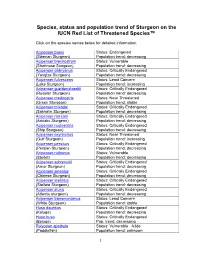
Species, Status and Population Trend of Sturgeon on the IUCN Red List of Threatened Species™
Species, status and population trend of Sturgeon on the IUCN Red List of Threatened Species™ Click on the species names below for detailed information: Acipenser baerii Status: Endangered (Siberian Sturgeon) Population trend: decreasing Acipenser brevirostrum Status: Vulnerable (Shortnose Sturgeon) Population trend: decreasing Acipenser dabryanus Status: Critically Endangered (Yangtze Sturgeon) Population trend: decreasing Acipenser fulvescens Status: Least Concern (Lake Sturgeon) Population trend: increasing Acipenser gueldenstaedtii Status: Critically Endangered (Russian Sturgeon) Population trend: decreasing Acipenser medirostris Status: Near Threatened (Green Sturgeon) Population trend: stable Acipenser mikadoi Status: Critically Endangered (Sakhalin Sturgeon) Population trend: decreasing Acipenser naccarii Status: Critically Endangered (Adriatic Sturgeon) Population trend: decreasing Acipenser nudiventris Status: Critically Endangered (Ship Sturgeon) Population trend: decreasing Acipenser oxyrinchus Status: Near Threatened (Gulf Sturgeon) Population trend: increasing Acipenser persicus Status: Critically Endangered (Persian Sturgeon) Population trend: decreasing Acipenser ruthenus Status: Vulnerable (Sterlet) Population trend: decreasing Acipenser schrenckii Status: Critically Endangered (Amur Sturgeon) Population trend: decreasing Acipenser sinensis Status: Critically Endangered (Chinese Sturgeon) Population trend: decreasing Acipenser stellatus Status: Critically Endangered (Stellate Sturgeon) Population trend: decreasing Acipenser -

PETITION to LIST Fifteen Species of Sturgeon UNDER the U.S
PETITION TO LIST Fifteen Species of Sturgeon UNDER THE U.S. ENDANGERED SPECIES ACT Submitted to the U.S. Secretary of Commerce, Acting through the National Oceanic and Atmospheric Administration and the National Marine Fisheries Service March 8, 2012 Petitioners WildEarth Guardians Friends of Animals 1536 Wynkoop Street, Suite 301 777 Post Road, Suite 205 Denver, Colorado 80202 Darien, Connecticut 06820 303.573.4898 203.656.1522 INTRODUCTION WildEarth Guardians and Friends of Animals hereby petitions the Secretary of Commerce, acting through the National Marine Fisheries Service (NMFS)1 and the National Oceanic and Atmospheric Administration (NOAA) (hereinafter referred as the Secretary), to list fifteen critically endangered sturgeon species as “threatened” or “endangered” under the Endangered Species Act (ESA) (16 U.S.C. § 1531 et seq.). The fifteen petitioned sturgeon species, grouped by geographic region, are: I. Western Europe (1) Acipenser naccarii (Adriatic Sturgeon) (2) Acipenser sturio (Atlantic Sturgeon/Baltic Sturgeon/Common Sturgeon) II. Caspian Sea/Black Sea/Sea of Azov (3) Acipenser gueldenstaedtii (Russian Sturgeon) (4) Acipenser nudiventris (Ship Sturgeon/Bastard Sturgeon/Fringebarbel Sturgeon/Spiny Sturgeon/Thorn Sturgeon) (5) Acipenser persicus (Persian Sturgeon) (6) Acipenser stellatus (Stellate Sturgeon/Star Sturgeon) III. Aral Sea and Tributaries (endemics) (7) Pseudoscaphirhynchus fedtschenkoi (Syr-darya Shovelnose Sturgeon/Syr Darya Sturgeon) (8) Pseudoscaphirhynchus hermanni (Dwarf Sturgeon/Little Amu-Darya Shovelnose/Little Shovelnose Sturgeon/Small Amu-dar Shovelnose Sturgeon) (9) Pseudoscaphirhynchus kaufmanni (False Shovelnose Sturgeon/Amu Darya Shovelnose Sturgeon/Amu Darya Sturgeon/Big Amu Darya Shovelnose/Large Amu-dar Shovelnose Sturgeon/Shovelfish) IV. Amur River Basin/Sea of Japan/Sea of Okhotsk (10) Acipenser mikadoi (Sakhalin Sturgeon) (11) Acipenser schrenckii (Amur Sturgeon) (12) Huso dauricus (Kaluga) V.