The Spatial Distribution of Post-Blast Condensed Phase Explosive
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Explosive Weapon Effectsweapon Overview Effects
CHARACTERISATION OF EXPLOSIVE WEAPONS EXPLOSIVEEXPLOSIVE WEAPON EFFECTSWEAPON OVERVIEW EFFECTS FINAL REPORT ABOUT THE GICHD AND THE PROJECT The Geneva International Centre for Humanitarian Demining (GICHD) is an expert organisation working to reduce the impact of mines, cluster munitions and other explosive hazards, in close partnership with states, the UN and other human security actors. Based at the Maison de la paix in Geneva, the GICHD employs around 55 staff from over 15 countries with unique expertise and knowledge. Our work is made possible by core contributions, project funding and in-kind support from more than 20 governments and organisations. Motivated by its strategic goal to improve human security and equipped with subject expertise in explosive hazards, the GICHD launched a research project to characterise explosive weapons. The GICHD perceives the debate on explosive weapons in populated areas (EWIPA) as an important humanitarian issue. The aim of this research into explosive weapons characteristics and their immediate, destructive effects on humans and structures, is to help inform the ongoing discussions on EWIPA, intended to reduce harm to civilians. The intention of the research is not to discuss the moral, political or legal implications of using explosive weapon systems in populated areas, but to examine their characteristics, effects and use from a technical perspective. The research project started in January 2015 and was guided and advised by a group of 18 international experts dealing with weapons-related research and practitioners who address the implications of explosive weapons in the humanitarian, policy, advocacy and legal fields. This report and its annexes integrate the research efforts of the characterisation of explosive weapons (CEW) project in 2015-2016 and make reference to key information sources in this domain. -

Influence of Different Polymeric Matrices on the Properties of Pentaerythritol Tetranitrate
Defence Science Journal, Vol. 71, No. 2, March 2021, pp. 177-184, DOI : 10.14429/dsj.71.16132 © 2021, DESIDOC Influence of Different Polymeric Matrices on the Properties of Pentaerythritol Tetranitrate Ahmed Elbeih*, Mahmoud Abdelhafiz, and Ahmed K. Hussein Military Technical College, Cairo, Egypt *E-mail: [email protected] ABSTRACT Six different polymeric matrices were fabricated to reduce the sensitivity of PETN (Pentaerythritol tetranitrate). The polymeric matrices used were individually based on Acrylonitrile butadiene rubber (NBR) softened by plasticizer, styrene-butadiene rubber (SBR) softened by oil, polymethyl methacrylate (PMMA) plasticised by dioctyl adipate (DOA), polydimethylsiloxane (PDMS), polyurethane matrix, and Fluorel binder. A computerised plastograph mixer was utilised for producing three polymer-bonded explosives (PETN-NBR, PETN-SBR, and PETN-PDMS) based on the non-aqueous method. A cast-cured method was used to prepare PBX based on polyurethane (PETN-HTPB), while the slurry technique was used to prepare beads of PETN coated by either fluorel binder (PETN-FL) or based on PMMA forming (PETN-PMMA). The heat of combustion and sensitivities were investigated. The velocity of detonation was measured, while the characteristics of the detonation wave were deduced theoretically by the EXPLO 5 (thermodynamic code). The ballistic mortar experiment was performed to determine the explosive strength. By comparing the results, it was found that PDMS has the highest influence on decreasing the impact sensitivity of PETN, while the cast cured PETN-HTPB has the lowest friction sensitivity. On the other side, PETN-FL has the highest detonation parameters with high impact sensitivity. Several relationships were verified and the matching between the measured results with the calculated ones was confirmed. -

May 25, 1965 M. A. COOK ETAL 3,185,017 METHOD of MAKING an EXPLOSIVE BOOSTER Original Filed July 13, 1959
May 25, 1965 M. A. COOK ETAL 3,185,017 METHOD OF MAKING AN EXPLOSIVE BOOSTER Original Filed July 13, 1959 FLS-1 INVENTORS AE1M at OOC AOWGLMS Al ACA 3,185,017 United States Patent Office Patented May 25, 1965 2 3,185,017 pressed pentolite (PETN-TNT), TNT, tetryl, tetrytol METHOD OF MAKING AN EXPLGSWE GOSTER (tetryl-TNT), cyclotol (RDX-TNT), composition B Melvin A. Cook and Dotigias E. Pack, 3: it lake City, (RDX-TNT-wax) and Ednatol (EDNA-TNT). The Utah, assig Rors to Intertanozzataia Researcia and Engi more inexpensive of these explosives are not detonatable eering Company, Inc., Saitake City, Utah, a cospera by Prinnacord, however. tion of Utah 5 Original application July 13, 1959, Ser. No. 826,539, how It has been found that PETN, PETN-TNT mixtures, Patent No. 3,637,453, dated June 5, 1932, Cisie at: RDX and cyclotol are detonated by Primacord, but their this application Oct. 24, 1960, Ser. No. 70,334 high cost and the relatively large quantities required make 2 Clairls. (C. 86-) it uneconomical to use them as boosters. O The improved boost cr of the invention generally com This invention relates to detonating means for ex prises a core of Primacord-sensitive explosive material plosives and more particularly to boosters for relatively sit trol inted by a compacted sheath of Primacord-insensi insensitive explosive compositions. This application is tive explosive imaterial of high brisance, said booster a division of our copending application Serial No. 826,539, having at cast one perforation extending through said filed July 13, 1959, now U.S. -

Detonation Cord, Detacord, Det
DDEPATMENT OF MINING ENGINEERING INTRODUCTION:- What are explosives? An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances. The potential energy stored in an explosive material may, for example, be chemical energy, such as nitro glycerine or grain dust DDEPATMENT OF MINING ENGINEERING pressurized gas, such as a gas cylinder or aerosol can nuclear energy, such as in the fissile isotopes uranium-235 and plutonium-239 Explosive materials may be categorized by the speed at which they expand. Materials that detonate (the front of the chemical reaction moves faster through the material than the speed of sound) are said to be "high explosives" and materials that deflagrate are said to be "low explosives". Explosives may also be categorized by their sensitivity. Sensitive materials that can be initiated by a relatively small amount of heat or pressure are primary explosives and materials that are relatively insensitive are secondary or tertiary explosives. A wide variety of chemicals can explode; a smaller number are manufactured specifically for the purpose of being used as explosives. The remainder are too dangerous, sensitive, toxic, expensive, DDEPATMENT OF MINING ENGINEERING unstable, or prone to decomposition or degradation over short time spans. DDEPATMENT OF MINING ENGINEERING HISTORY:- The use of explosives in mining goes back to the year 1627, when gunpowder was first used in place of mechanical tools in the Hungarian (now Slovak) town of Banská Štiavnica. -

Guide for the Selection of Commercial Explosives Detection Systems For
2.5.3.8 EXPRAY Field Test Kit EXPRAY is a unique, aerosol-based field test kit for the detection of what the manufacturer refers to as Group A explosives (TNT, DNT, picric acid, etc.), Group B explosives (Semtex H, RDX, PETN, NG, smokeless powder, etc.), and compounds that contain nitrates that are used in improvised explosives. Detection of explosive residue is made by observing a color change of the test paper. EXPRAY can be used in a variety of applications, and although in some aspects it does not perform as well as many of the other trace detectors discussed in this section, it costs only $250. This very low cost, coupled with simplicity and ease of use, may make it of interest to many law enforcement agencies (see the EXPRAY kit in fig. 13). The EXPRAY field kit2 is comprised of the following items: - one can of EXPRAY-1 for Group A explosives, - one can of EXPRAY-2 for Group B explosives, - one can of EXPRAY-3 for nitrate-based explosives (ANFO, black powder, and commercial and improvised explosives based on inorganic nitrates), - special test papers which prevent cross contamination. Figure 13. Photo of the EXPRAY Field Test Kit for explosives Initially, a suspected surface (of a package, a person’s clothing, etc.) is wiped with the special test paper. The paper is then sprayed with EXPRAY-1. The appearance of a dark violet-brown color indicates the presence of TNT, a blue-green color indicates the presence of DNT, and an orange color indicates the presence of other Group A explosives. -

Explosive Characteristics and Performance Larry Mirabelli Design Factors for Chemical Crushing
Explosive Characteristics and Performance Larry Mirabelli Design Factors for Chemical Crushing Controllable EXPLOSIVEEXPLOSIVE Uncontrollable GEOLOGYROCK CONFINEMENT DISTRIBUTION Course Agenda What is an Explosive Explosive Types What are best to fuel “chemical crusher” Explosive Properties Explosive types Characteristics General Application Explosive selection to meet blasting objectives What is an Explosive Intimate mixture of fuel and oxidizer. Can be a molecular solid, liquid or gas and/or mixtures of them. When initiated reacts very quickly to form heat, solids and gas. Violent exothermic oxidation-reduction reaction. Detonates instead of burning. Rate of reaction is its detonation velocity. Explosive Types Main Explosive Charge Explosive for use in primer make up. Initiation System Explosive Types – Main Explosive Charge Bulk Explosive Blasting Agent, 1.5 D (not detonator sensitive) • Repumpable – Emulsion (available with field density adjustment and/or homogenization) • Repumpable ANFO Blend – Emulsion (available with field density adjustment) • Heavy ANFO Blend – Emulsion • ANFO For fueling chemical crusher, application flexibility for changing design is best decision. Explosive Types – Main Explosive Charge Packaged Explosive Explosive, 1.1D (detonator sensitive) • Emulsion • Dynamite Blasting Agent, 1.5 D (not detonator sensitive) • Emulsion • Water Gel • WR ANFO • ANFO For fueling chemical crusher, it is best to optimize explosive distribution. Explosive Types Explosive for use in primer make up. Explosive, 1.1D (detonator sensitive) • Cast Booster • Dynamite • Emulsion For fueling chemical crusher, cast booster is recommended explosive for primer make up. Explosive Types Initiation System Electronic Non Electric Electric For fueling chemical crusher, Electronic Detonator is recommended. Explosive Properties Safety properties Characterize transportation, storage, handling and use Physical properties Characterize useable applications and loading equipment requirements. -
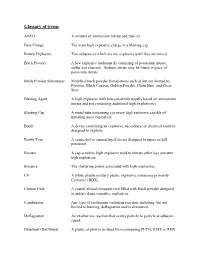
TWGFEX Glossary of Terms
Glossary of terms ANFO A mixture of ammonium nitrate and fuel oil. Base Charge The main high explosive charge in a blasting cap. Binary Explosive Two substances which are not explosive until they are mixed. Black Powder A low explosive traditionally consisting of potassium nitrate, sulfur and charcoal. Sodium nitrate may be found in place of potassium nitrate. Black Powder Substitutes Modified black powder formulations such as but not limited to: Pyrodex, Black Canyon, Golden Powder, Clean Shot, and Clear Shot. Blasting Agent A high explosive with low-sensitivity usually based on ammonium nitrate and not containing additional high explosive(s). Blasting Cap A metal tube containing a primary high explosive capable of initiating most explosives. Bomb A device containing an explosive, incendiary, or chemical material designed to explode. Booby Trap A concealed or camouflaged device designed to injure or kill personnel. Booster A cap sensitive high explosive used to initiate other less sensitive high explosives. Brisance The shattering power associated with high explosives. C4 A white pliable military plastic explosive containing primarily Cyclonite (RDX). Cannon Fuse A coated, thread-wrapped cord filled with black powder designed to initiate flame-sensitive explosives. Combustion Any type of exothermic oxidation reaction, including, but not limited to burning, deflagration and/or detonation. Deflagration An exothermic reaction that occurs particle to particle at subsonic speed. Detasheet (Det Sheet) A plastic explosive in sheet form containing PETN, HMX or RDX. Detonation An exothermic reaction that propagates a shockwave through an explosive at supersonic speed (greater than 3300ft/sec). Detonation Cord (Det-Cord) A plastic/fiber wrapped cord containing a core of PETN or RDX. -
Render-Safe Impact Dynamics of the M557 Fuze G.A. Gazonas U.S. Army
Transactions on the Built Environment vol 32, © 1998 WIT Press, www.witpress.com, ISSN 1743-3509 Render-safe impact dynamics of the M557 fuze G.A. Gazonas U.S. Army Research Laboratory Weapons and Materials Research Directorate Aberdeen Proving Ground, MD 21005, USA e-mail: [email protected] Abstract This paper outlines the results of a combined experimental and computational study which investigates the transient structural response of the M557 point detonating fuze subjected to low-speed (200 m/s) oblique impact by a hardened steel projectile. The problem is of interest to the explosive ordnance disposal (EOD) community as it is a method that is currently used to "render-safe" ordnance and other explosive devices found in the field [1]. An inert M557 fuze is instrumented with a low mass (1.5 gram), 60 kilo-g uniaxial accelerometer and subjected to oblique impact by launching a 300 gram projectile from a 4-in airgun. The transient structural response of the fuze is compared to the predicted response using the Lagrangian finite element code DYNA3D. Peak accelerations measured in two tests average 35 kilo-g whereas DYNA3D predicts a 40 to 50 kilo-g peak acceleration depending upon the amount of prescribed damping. Both measured and computed acceleration histories are Fourier transformed and the estimated spectral response at the base of the fuze is shown to be dependent upon the failure strength of the flash tube. 1 Introduction The explosive ordnance disposal (EOD) community utilizes a variety of devices to disarm or "render-safe" ordnance and other explosive devices found in the field. -

Theoretical Principles for Successful Littoral Special Operations
Calhoun: The NPS Institutional Archive Theses and Dissertations Thesis and Dissertation Collection 2016-06 Maritime SOF in the littorals: theoretical principles for successful littoral special operations Grimeland, Torbjorn Monterey, California: Naval Postgraduate School http://hdl.handle.net/10945/49475 NAVAL POSTGRADUATE SCHOOL MONTEREY, CALIFORNIA THESIS MARITIME SOF IN THE LITTORALS: THEORETICAL PRINCIPLES FOR SUCCESSFUL LITTORAL SPECIAL OPERATIONS by Torbjorn Grimeland Oscar van der Veen June 2016 Thesis Advisor: Kalev I. Sepp Second Reader: Ian Rice Approved for public release; distribution is unlimited THIS PAGE INTENTIONALLY LEFT BLANK REPORT DOCUMENTATION PAGE Form Approved OMB No. 0704-0188 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instruction, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to Washington headquarters Services, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202-4302, and to the Office of Management and Budget, Paperwork Reduction Project (0704-0188) Washington DC 20503. 1. AGENCY USE ONLY 2. REPORT DATE 3. REPORT TYPE AND DATES COVERED (Leave blank) June 2016 Master’s thesis 4. TITLE AND SUBTITLE 5. FUNDING NUMBERS MARITIME SOF IN THE LITTORALS: THEORETICAL PRINCIPLES FOR SUCCESSFUL LITTORAL SPECIAL OPERATIONS 6. AUTHOR(S) Torbjorn Grimeland and Oscar van der Veen 7. PERFORMING ORGANIZATION NAME(S) AND ADDRESS(ES) 8. PERFORMING Naval Postgraduate School ORGANIZATION REPORT Monterey, CA 93943-5000 NUMBER 9. -

Surface Mining
Surface Mining Products, Services and Reference Guide Contents n Services 4 n Bulk Explosives 5 n Packaged Explosives 21 n Initiation Systems 29 n Blasting Accessories 45 n Explosive Delivery Systems 47 n Reference 49 Why do companies come to Dyno Nobel for groundbreaking solutions? Dyno Nobel is a global leader in commercial explosives and blast design. One of our core strengths is our understanding of the processes and challenges that impact on the mining industry. We leverage our decades of industry experience to meet and exceed customer expectations. We can draw upon our global resources to provide our customers with fully tailored solutions that can make a positive difference to your project. Our team work with mining companies to solve highly complex problems and they can help you too. Dyno Nobel has the people, the resources and the experience to deliver Groundbreaking Performance®. Dyno Nobel is a business of Incitec Pivot Limited (IPL), an S&P/ASX Top 50 company listed on the Australian Securities Exchange (ASX). Dyno Nobel Asia Pacific provides a full range of industrial explosives, related products and services to customers in Australia, Indonesia, Papua New Guinea, Turkey, Latin America, the United States and Canada. Services Consulting services Tailored training courses DynoConsult®, the specialist consulting division of Dyno Nobel backs up its innovative product offering with Dyno Nobel, aims to improve customer performance. world class support for our customers. Its resources include a team of experienced professionals To reinforce safe practices and upskill technical with local and international experience in open cut proficiency, Dyno Nobel offers a full range of training mining processes. -

IMEMTS 2006 Booster Explosives Paper
EXPLOSIVE BOOSTER SELECTION CRITERIA FOR INSENSITIVE MUNITIONS APPLICATIONS Ron Hollands BAE SYSTEMS RO Defence, Glascoed, Usk, Monmouthshire, NP15 1XL, UK. Tel: +44-(0)1291-674181; Fax: +44-(0)1291-674103; E-mail: [email protected] Peter Barnes, Ron Moss and Mike Sharp Defence Ordnance Safety Group, MOD Abbey Wood, Bristol, BS34 8JH, UK. Introduction Boosters play an essential role in the safe functioning of the majority of explosive-filled ordnance by propagating and magnifying the detonation wave from initiator to main charge. Booster explosives formulated for Insensitive Munitions (IMs) applications must fulfil a number of exacting and often apparently conflicting requirements. For example, the booster must be shock sensitive enough to reliably take over from the detonator and at the same time must possess reduced vulnerability towards hazardous stimuli as represented by the threats described in STANAG 4439. Consistent performance and survivability under in-service conditions must also be maintained throughout the lifecycle of the munition. This necessitates preservation of physical integrity, thermal stability and resistance to ageing, particularly if the explosive is to be subjected to high–g environments during launch or retardation. Selection Criteria This paper describes the rationale and methodology adopted in the United Kingdom for the assessment and selection of candidate pressable booster formulations as part of a systems approach to meeting IM requirements. The Insensitive Munitions Assessment Panel (IMAP) reviews available data on design features, such as boosters, beginning at an early stage in a project’s life. These reviews are undertaken to assess the IM compatibility of proposed design options and choices of energetic materials with a view to defining the IM signature for the munition. -

FM 5-250: Explosives and Demolitions
FM 5-250 HEADQUARTERS Field Manual DEPARTMENT OF THE ARMY 5-250 Washington, DC, 15 June 1992 i FM 5-250 ii FM 5-250 iii FM 5-250 iv FM 5-250 v FM 5-250 vi FM 5-250 vii FM 5-250 viii FM 5-250 ix FM 5-250 x FM 5-250 xi FM 5-250 xii FM 5-250 xiii FM 5-250 xiv FM 5-250 xv FM 5-250 xvi FM 5-250 xvii FM 5-250 xviii FM 5-250 xix FM 5-250 xx FM 5-250 xxii FM 5-250 xxi FM 5-250 xxiv FM 5-250 xxiii FM 5-250 xxv FM 5-250 xxvi FM 5-250 xxvii FM 5-250 Preface The purpose of this manual is to provide technical information on explosives used by United States military forces and their most frequent applications. This manual does not discuss all applications but presents the most current information on demolition procedures used in most situations. If used properly, explosives serve as a combat multiplier to deny maneuverability to the enemy. Focusing mainly on these countermobility operations, this manual provides a basic theory of explosives, their characteristics and common uses, formulas for calculating various types of charges, and standard methods of priming and placing these charges. When faced with unusual situations, the responsible engineer must either adapt one of the recommended demolition methods or design the demolition from basic principles presented in this and other manuals. The officer in charge must maintain ultimate responsibility for the demolition design, ensuring the safe and efficient application of explosives.