A Closer Look: Documentation and Coding for Cardiac Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Follow-Up of 134 Pediatric Patients with Wolff-Parkinson-White Pattern: Natural Outcome and Medical Treatment
ORIGINAL ARTICLE Follow-up of 134 Pediatric Patients with Wolff-Parkinson-White Pattern: Natural Outcome and Medical Treatment AMALIA N. STEFANI, GABRIELA R. DAL FABBRO, MARÍA J. BOSALEH, ROBERTH VÁSQUEZ, GUSTAVO A. COSTA, RICARDO SPERANZA, JORGE L. GENTILE, CLAUDIO DE ZULOAGAMTSAC Received: 01/03/2013 ABSTRACT Accepted: 01/03/2013 Address for reprints: Objective Amalia N. Stefani The aim of the study was to evaluate the outcome of a pediatric population with Almafuerte 1722 ventricular pre-excitation pattern, supraventricular tachycardia, atrial fibrillation, (1650) San Martín, cardiomyopathies, mortality and medical treatment. Pcia. de Buenos Aires e-mail: [email protected] Methods From 1976 to 2011, a descriptive observational study was conducted on patients with ventricular pre-excitation in the electrocardiogram. All patients underwent an echocardiogram, 101(75.3%) Holter monitoring, and 69 (51.5%) an ergometric test. Radiofrequency ablation was performed in selected patients. Data were expressed as mean and standard deviation. Results The study population consisted of 134 patients; 80 (59.7%) were male. Age at diag- nosis ranged from 2 days to 18 years (mean 6.5±5 years). Clinical follow-up lasted 1 month to 20 years (mean 3.6±3.9 years). Thirty five patients (26.1%) consulted for supraventricular tachycardia, 16 (11.9%) for ventricular pre-excitation, and the remaining 83 patients (61.9%) for other abnormalities. Seventy-six patients (56.7%) had left conduction pathway and 3 patients double conduction pathway. Sixteen pa- tients (11.9%) presented supraventricular tachycardia during follow-up. Overall, 51 patients (38%) had orthodromic tachycardia at 6.3±5.8 years, 10 patients during the neonatal period. -

Cardiac Involvement in COVID-19 Patients: a Contemporary Review
Review Cardiac Involvement in COVID-19 Patients: A Contemporary Review Domenico Maria Carretta 1, Aline Maria Silva 2, Donato D’Agostino 2, Skender Topi 3, Roberto Lovero 4, Ioannis Alexandros Charitos 5,*, Angelika Elzbieta Wegierska 6, Monica Montagnani 7,† and Luigi Santacroce 6,*,† 1 AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Coronary Unit and Electrophysiology/Pacing Unit, Cardio-Thoracic Department, Policlinico University Hospital of Bari, 70124 Bari, Italy; [email protected] 2 AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Cardiac Surgery, Policlinico University Hospital of Bari, 70124 Bari, Italy; [email protected] (A.M.S.); [email protected] (D.D.) 3 Department of Clinical Disciplines, School of Technical Medical Sciences, University of Elbasan “A. Xhuvani”, 3001 Elbasan, Albania; [email protected] 4 AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Clinical Pathology Unit, Policlinico University Hospital of Bari, 70124 Bari, Italy; [email protected] 5 Emergency/Urgent Department, National Poisoning Center, Riuniti University Hospital of Foggia, 71122 Foggia, Italy 6 Department of Interdisciplinary Medicine, Microbiology and Virology Unit, University of Bari “Aldo Moro”, Piazza G. Cesare, 11, 70124 Bari, Italy; [email protected] 7 Department of Biomedical Sciences and Human Oncology—Section of Pharmacology, School of Medicine, University of Bari “Aldo Moro”, Policlinico University Hospital of Bari, p.zza G. Cesare 11, 70124 Bari, Italy; [email protected] * Correspondence: [email protected] (I.A.C.); [email protected] (L.S.) † These authors equally contributed as co-last authors. Citation: Carretta, D.M.; Silva, A.M.; D’Agostino, D.; Topi, S.; Lovero, R.; Charitos, I.A.; Wegierska, A.E.; Abstract: Background: The widely variable clinical manifestations of SARS-CoV2 disease (COVID-19) Montagnani, M.; Santacroce, L. -

Non Commercial Use Only
Cardiogenetics 2017; volume 7:6304 Sudden death in a young patient with atrial fibrillation Case Report Correspondence: María Angeles Espinosa Castro, Inherited Cardiovascular Disease A 22-year-old man suffered a sudden Program, Cardiology Department, Gregorio María Tamargo, cardiac arrest without previous symptoms Marañón Hospital, Dr. Esquerdo, 46, 28007, María Ángeles Espinosa, while he was at rest, waiting for a subway Madrid, Spain. Víctor Gómez-Carrillo, Miriam Juárez, train. Cardiopulmonary resuscitation was Tel.: +34.91.586.82.90. immediately started using an Automated E-mail: [email protected] Francisco Fernández-Avilés, External Defibrillation that identified the Raquel Yotti Key words: KCNQ1; mutation; channelopa- presence of ventricular fibrillation and thy; sudden cardiac death; atrial fibrillation. Inherited Cardiovascular Disease delivered a shock. Return of spontaneous Program, Cardiology Department, circulation was achieved after three Contributions: MT, acquisition and interpreta- Gregorio Marañón Hospital, Madrid, attempts, being atrial fibrillation (AF) the tion of data for the work, ensuring that ques- Spain patient’s rhythm at this point (Figure 1). tions related to the accuracy or integrity of any He was admitted to our Cardiovascular part of the work is appropriately investigated Intensive Care Unit and therapeutic and resolved; MAE, conception of the work, hypothermia was performed over a period critical revision of the intellectual content, final approval of the version to be published, Abstract of 24 h. After completing hypothermia, ensuring that questions related to the accuracy rewarming, and another 24 h of controlled of any part of the work is appropriately inves- Sudden cardiac death (SCD) in young normothermia the patient awakened with no tigated and resolved; VG-C, acquisition and patients without structural heart disease is residual neurologic damage. -
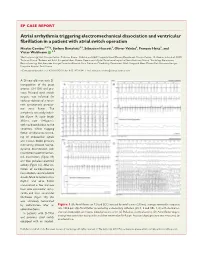
Atrial Arrhythmia Triggering Electromechanical Dissociation And
EP CASE REPORT ....................................................................................................................................................... Atrial arrhythmia triggering electromechanical dissociation and ventricular fibrillation in a patient with atrial switch operation Nicolas Combes1,2,3*, Stefano Bartoletti1,2,Se´bastien Hascoet€ 3, Olivier Vahdat2, Franc¸ois Heitz2, and Victor Waldmann 4,5 1Electrophysiology Unit, Clinique Pasteur, Toulouse, France; 2Pediatric and Adult Congenital Heart Disease Department, Clinique Pasteur, 45, Avenue de Lombez, 31076 Toulouse, France; 3Pediatric and Adult Congenital Heart Disease Department, Hoˆpital Marie Lannelongue, Le Plessis-Robinson, France; 4Cardiology Department, Electrophysiology Unit, European Georges Pompidou Hospital, Paris, France; and 5Cardiology Department, Adult Congenital Heart Disease Unit, European Georges Pompidou Hospital, Paris, France * Corresponding author. Tel: 133 562213131; fax: 133 562211641. E-mail address: [email protected] A 26-year-old man with D- transposition of the great arteries (D-TGA) and pre- vious Mustard atrial switch surgery was referred for catheter ablation of a recur- rent symptomatic paroxys- mal atrial flutter. The arrhythmia was easily induci- ble (Figure 1A, cycle length 310 ms, rate 194 b.p.m.), with rapid conduction to the ventricles. While mapping flutter, simultaneous record- ing of endocardial signals and invasive blood pressure monitoring showed haemo- dynamic deterioration with intermittent electromechan- ical dissociation (Figure 1B) and then pulseless electrical activity (Figure 1C). After ini- tiation of cardiopulmonary resuscitation, several electric shocks failed to restore sinus rhythm and atrial flutter transitioned a few minutes later into ventricular tachy- cardia and then ventricular fibrillation (Figure 1D); this was ultimately terminated by defibrillation after an Figure 1 (A) Atrial flutter on 12-lead ECG induced by atrial bursts (240 ms), average ventricular response adrenaline bolus. -

Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: the Role of Multimodality Imaging to Detect High-Risk Features
diagnostics Review Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality Imaging to Detect High-Risk Features Anna Giulia Pavon 1,2,*, Pierre Monney 1,2,3 and Juerg Schwitter 1,2,3 1 Cardiac MR Center (CRMC), Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland; [email protected] (P.M.); [email protected] (J.S.) 2 Cardiovascular Department, Division of Cardiology, Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland 3 Faculty of Biology and Medicine, University of Lausanne (UniL), 1100 Lausanne, Switzerland * Correspondence: [email protected]; Tel.: +41-775-566-983 Abstract: Mitral valve prolapse (MVP) was first described in the 1960s, and it is usually a benign condition. However, a subtype of patients are known to have a higher incidence of ventricular arrhythmias and sudden cardiac death, the so called “arrhythmic MVP.” In recent years, several studies have been published to identify the most important clinical features to distinguish the benign form from the potentially lethal one in order to personalize patient’s treatment and follow-up. In this review, we specifically focused on red flags for increased arrhythmic risk to whom the cardiologist must be aware of while performing a cardiovascular imaging evaluation in patients with MVP. Keywords: mitral valve prolapse; arrhythmias; cardiovascular magnetic resonance Citation: Pavon, A.G.; Monney, P.; Schwitter, J. Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality 1. Mitral Valve and Arrhythmias: A Long Story Short Imaging to Detect High-Risk Features. In the recent years, the scientific community has begun to pay increasing attention Diagnostics 2021, 11, 683. -

Constrictive Pericarditis Causing Ventricular Tachycardia.Pdf
EP CASE REPORT ....................................................................................................................................................... A visually striking calcific band causing monomorphic ventricular tachycardia as a first presentation of constrictive pericarditis Kian Sabzevari 1*, Eva Sammut2, and Palash Barman1 1Bristol Heart Institute, UH Bristol NHS Trust UK, UK; and 2Bristol Heart Institute, UH Bristol NHS Trust UK & University of Bristol, UK * Corresponding author. Tel: 447794900287; fax: 441173425926. E-mail address: [email protected] Introduction Constrictive pericarditis (CP) is a rare condition caused by thickening and stiffening of the pericar- dium manifesting in dia- stolic dysfunction and enhanced interventricu- lar dependence. In the developed world, most cases are idiopathic or are associated with pre- vious cardiac surgery or irradiation. Tuberculosis remains a leading cause in developing areas.1 Most commonly, CP presents with symptoms of heart failure and chest discomfort. Atrial arrhythmias have been described as a rare pre- sentation, but arrhyth- mias of ventricular origin have not been reported. Figure 1 (A) The 12 lead electrocardiogram during sustained ventricular tachycardia is shown; (B and C) Case report Different projections of three-dimensional reconstructions of cardiac computed tomography demonstrating a A 49-year-old man with a striking band of calcification around the annulus; (D) Carto 3DVR mapping—the left hand panel (i) demonstrates a background of diabetes, sinus beat with late potentials at the point of ablation in the coronary sinus, the right hand panel (iii) shows the hypertension, and hyper- pacemap with a 89% match to the clinical tachycardia [matching the morphology seen on 12 lead ECG (A)], and cholesterolaemia and a the middle panel (ii) displays the three-dimensional voltage map. -

Mitral Valve Disease in Dogs- Truly Epic Kristin Jacob, DVM, DACVIM CVCA Cardiac Care for Pets Towson, MD
Mitral Valve Disease in Dogs- Truly Epic Kristin Jacob, DVM, DACVIM CVCA Cardiac Care for Pets Towson, MD Chronic degenerative mitral valvular disease (DMVD) is the most common heart disease in dogs. In dogs with congestive heart failure, 75% have mitral regurgitation/degenerative valvular disease. Almost all have tricuspid regurgitations as well, but clinically the mitral regurgitation is the most significant. Degenerative valvular disease is most common in small breed dogs and more common in males than females. DMVD has been proven to be inherited in the Cavalier King Charles Spaniel and the Dachshund but several other breeds are predisposed, such as Bichons, poodles, Chihuahuas, Miniature Schnauzers, Boston Terriers. The cause of degenerative valvular disease remains unknown. However, the valve changes occur due to a destruction of collagen, deposition of mucopolysaccharide in the spongiosa and fibrosa layer of mitral valve, which also affects chordae tendinea. These changes prevent effective coaptation of the valve leaflets leading to progressive mitral regurgitation. Mitral regurgitation (MR) leads to decreasing forward output and increasing left atrial and left ventricular dilation and remodeling as well as activation of the neurohormonal systems. Typically, patients will have 2-3 years in asymptomatic phase (time between when heart murmur detected) until symptoms develop - congestive heart failure (CHF). We can detect progressive heart enlargement 6-12 months prior to the development of CHF. This means that we have a fairly long time period to intervene with therapy and alter the progression of the disease - we know what’s coming! Eventually left atrial pressure increases sufficiently and pulmonary congestion develops leading to symptoms of CHF and can also lead to pulmonary hypertension. -

Atrial Flutter Patient Information
AF A Atrial flutter patient information Providing information, support and access to established, new or innovative treatments for atrial fibrillation www.afa-international.org Registered Charity No. 1122442 Glossary Antiarrhythmic drugs Drugs used to restore the Contents normal heart rhythm Introduction Anticoagulant A group of drugs which help to thin the blood and prevent AF-related stroke What is atrial flutter? Arrhythmia A heart rhythm disorder What causes atrial flutter? Atrial flutter A rhythm disorder characterised by a rapid but regular atrial rate although not as high as What are the atrial fibrillation symptoms of atrial flutter? A therapy to treat arrhythmias Cardioversion How do I get to which uses a transthoracic electrical shock to revert see the right doctor the heart back into a normal rhythm to treat my atrial flutter? Catheter ablation A treatment by which the small area inside the heart which has been causing atrial What are the risks flutter is destroyed of atrial flutter? Echocardiogram An image of the heart using Diagnosis and echocardiography or soundwave-based technology. treatment An echocardiogram (nicknamed ‘echo’) shows a three-dimensional shot of the heart Treatment of atrial flutter Electrocardiogram (ECG) A representation of the heart’s electrical activity in the form of wavy lines. Drug treatments An ECG is taken from electrodes on the skin surface Stroke prevention The inability (failure) of the heart to Heart failure Non-drug treatments pump sufficient oxygenated blood around the body to meet physiological requirements 2 Introduction Atrial flutter is a relatively common heart rhythm The heart disturbance encountered by doctors, although not and normal as common as atrial fibrillation (AF). -

Pacemaker Syndrome Pacemaker Therapy Has Become an Important Therapeutic Option for Patients with Heart Rhythm Conditions Worldwide
360 Cardiology Pacemaker syndrome Pacemaker therapy has become an important therapeutic option for patients with heart rhythm conditions worldwide. Te number of elderly patients needing pacemakers is on the increase due to an ageing population worldwide. Pacemaker syndrome consists of the cardiovascular signs and symptoms of heart failure and hypotension induced by right ventricular (RV) pacing. Dr Satnam Singh Research Registrar, University of Aberdeen, Level 3, Polwarth building, Aberdeen email [email protected] Pacemaker syndrome is a term syndrome occurring in dual trial was a single blinded study proposed in 1979 by Erbel and chamber modes.5,6 It can even enrolling around 2000 patients refers to symptoms and signs in occur with AAI pacing with long with sick sinus syndrome. the pacemaker patient caused by PR intervals. All patients were implanted inadequate timing of atrial and dual chamber pacemakers ventricular contractions.1 It was first programmed to VVIR or DDDR described in 1969 by Mitsui et al2 as Incidence before implantation. Pacemaker an iatrogenic disease characterised syndrome was a secondary by the disappearance of symptoms Te overall incidence of pacemaker endpoint studied. Severe with restoration of atrioventricular syndrome is very difficult to pacemaker syndrome developed synchrony (AV synchrony). estimate but is about 20% in a in nearly 20% of VVIR-paced It means if atria and ventricles landmark trial called the Mode patients and improved with contract at appropriate timings (as Selection Trial (MOST).7 It occurs reprogramming to the dual- close to physiological), pacemaker with equal frequency in both sexes chamber pacing mode. syndrome can be prevented. -

What Are Premature Ventricular Contractions?
What Are Premature Ventricular Contractions? Premature ventricular contractions (VPCs or PVCs) are irregular beats that originate from the heart’s pumping chambers (ventricles). These beats interrupt the normal regular (sinus) rhythm, and result in irregularity to the heart rhythm. Although single PVCs are not life- threatening, they may indicate the presence of underlying heart disease, or when they become more severe can cause rapid and dangerous increases in heart rate (ventricular tachycardia). WHAT CAUSES PREMATURE VENTRICULAR CONTRACTIONS? PVCs can occur secondary to a variety of causes, most concerning being cardiac disease. PVCs are most commonly seen in dogs with Dilated Cardiomyopathy, but can also occur in the later stages of disease with Myxomatous Mitral Valve Degeneration. PVCs and other ventricular arrhythmias can also with no evidence of underlying structural heart disease. The workup for PVCs can be frustrating, because PVCs can also occur secondary to conditions unrelated to the heart. Most commonly they can occur secondary to gastrointestinal disease, systemic disease, and pain. HOW ARE PREMATURE VENTRICULAR CONTRACTIONS DIAGNOSED? Most commonly, your veterinarian will hear an irregular heart rhythm and recommend an electrocardiogram (ECG) to assess the heart rhythm. The ECG will enable your veterinarian to determine whether the irregular rhythm is due to PVCs. Following this diagnosis, there are several additional recommended tests. TESTING Following this diagnosis, there are several additional recommended tests. ECHOCARDIOGRAPHY Echocardiography (heart ultrasound) is recommended to make sure that there is no evidence of structural cardiac disease causing the abnormal heart rhythm. 24-HOUR HOLTER Single PVCs usually do not require treatment, however a Holter monitor will determine whether the frequency or severity of the MONITOR PVCs warrant anti-arrhythmic medication, and make sure that there is no evidence of ventricular tachycardia, which is a life threatening heart rhythm. -

ARIC Cohort Stroke Form Instructions STR, VERSION F Qxq, 01/23/2020
STRF Instructions (QxQs) This table summarizes changes to the STRF QxQ as of 01/23/2020 Question in STRF QxQ Description of Changes in STRF QXQ Section G., pg. 2 Record “2 days” when seeing “few days” Item 17., pg. 5 Remove ICD-10 codes (I65.x, I66.x, or I67.x) Item 21, pg. 6 Clarifications made on how to record this question if there are multiple TIAs Item 29.b., pg. 9 Clarifications made on how to record intracardia thrombus Item 29.c., pg. 9 Clarifications made on how to record atrial fibrillation or flutter Item 29.d., pg. 9 Clarifications made on how to record valvular heart disease Items 29.j.& 29.k., pg. 11 Remove “amyloid angiopathy or amyloidosis” from the “central nervous system” list Item 30.e. Clarifications made on how to record therapy with heparin or warfarin Item 44, pg. 15 Added “hemisensory” to the synonym list Items 48.d.1.& 48.e.1., pg. 18 Clarifications made on how to record stenosis Items 49 & 50, pg. 19 Clarifications made on how to record CT Scan Items 49.d.& 50.d., pg. 19 Clarifications made on how to record CT diagnosis Item 52.d., pg. 21 Clarifications made on how to record MRI diagnosis Items 53.c.1. and 53.d.1., pg. 21 Clarifications made on how to record the exact stenosis for right and left internal carotid artery of the neck Appendix B, pg. 27 and pg. 28 Clarifications made to the instructions regarding unrelated pathology or findings Appendix C, pg. 30 Updates made to hospital codes Appendix G, pg. -

Clinical Manifestation and Survival of Patients with I Diopathic Bilateral
ORIGINAL ARTICLE Clinical Manifestation and Survival of Patients with Mizuhiro Arima, TatsujiI diopathicKanoh, Shinya BilateralOkazaki, YoshitakaAtrialIwama,DilatationAkira Yamasaki and Sigeru Matsuda Westudied the histories of eight patients who lacked clear evidence of cardiac abnormalities other than marked bilateral atrial dilatation and atrial fibrillation, which have rarely been dis- cussed in the literature. From the time of their first visit to our hospital, the patients' chest radio- graphs and electrocardiograms showed markedly enlarged cardiac silhouettes and atrial fibrilla- tion, respectively. Each patient's echocardiogram showed a marked bilateral atrial dilatation with almost normal wall motion of both ventricles. In one patient, inflammatory change was demonstrated by cardiac catheterization and endomyocardial biopsy from the right ventricle. Seven of our eight cases were elderly women.Over a long period after the diagnosis of cardiome- galy or arrhythmia, diuretics or digitalis offered good results in the treatment of edema and congestion in these patients. In view of the clinical courses included in the present study, we conclude that this disorder has a good prognosis. (Internal Medicine 38: 112-118, 1999) Key words: cardiomegaly, atrial fibrillation, elder women,good prognosis Introduction echocardiography. The severity of mitral and tricuspid regur- gitation was globally assessed by dividing into three equal parts Idiopathic enlargement of the right atrium was discussed by the distance from the valve orifice. The regurgitant jet was de- Bailey in 1955(1). This disorder may be an unusual congenital tected on color Doppler recording in the four-chamber view malformation. A review of the international literature disclosed and classified into one of the three regions (-: none, +: mild, that although several cases have been discussed since Bailey's ++:moderate, +++: severe).