Monophyly of the Falconiformes Based On
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Secretary Bird
Secretary Birds are Raptors, or Birds of Prey, they are distantly related to vultures and hawks! They have all of the features of a bird of prey too, including forward facing eyes for hunting, strong talons to grab their prey, and a large hooked beak for eating! Secretary Birds do fly, but they spend of most of their day hunting by walking on the ground. Their long legs are perfect to help them see over the tall grass of their Savanna habitat. Deadly Stomp Secretary Birds have two hunting strategies. They either strike with their powerful beak or they stomp the prey with their feet! They hunt small birds, mammals, and reptiles like snakes and lizards. They are even known to hunt venomous snakes! The Little Rock Zoo works with the Secretary Bird Species Survival Plan, which helps to protect this amazing species in zoos and in the wild! Want to Learn more? Check out these links and activities! Nat Geo Wild: https://www.youtube.com/watch?v=kQckuAwNbFs Nat Geo Kids: https://www.youtube.com/watch?v=7itwHJiNRz4 Kruger National Park (South Africa): http://www.krugerpark.co.za/africa_secretary_bird.html Edge of Existence (ZSL): http://www.edgeofexistence.org/species/secretarybird/ San Diego Zoo: https://animals.sandiegozoo.org/index.php/animals/secretary-bird Secretary Birds are amazing Birds of Prey that spend most of their time on the ground rather than flying to find their food! Cut out the puzzle pieces to make your very own Secretary Bird Puzzle! Photo by Karen Caster . -

198 Pursuit and Capture of a Ring-Billed Gull by Bald
198 Florida Field Naturalist 28(4):198-200, 2000. PURSUIT AND CAPTURE OF A RING-BILLED GULL BY BALD EAGLES ANDREW W. KRATTER1 AND MARY K. HART2 1Florida Museum of Natural History, P.O. Box 117800, University of Florida Gainesville, Florida 3261; email: kratter@flmnh.ufl.edu 2Department of Fisheries and Aquatic Sciences, University of Florida Gainesville, Florida, 32611 Bald Eagles (Haliaeetus leucocephalus) are opportunistic hunters that employ a num- ber of techniques to capture a wide variety of prey (Bent 1937, Brown and Amadon 1968, Sherrod et al. 1976, McEwan and Hirth 1980). These eagles are known to occasionally pursue prey, including flying birds, in pairs or larger groups (McIlhenny 1932, Sherrod et al. 1976, Folk 1992). Here we report three Bald Eagles giving a prolonged chase of a Ring- billed Gull (Larus delawarensis), which resulted in the gull’s capture by one of the eagles. At approximately 1500 on 12 December 1998, we were kayaking east across Newnan’s Lake in eastern Alachua County, Florida, when we noticed a group of approximately 50 Ring-billed Gulls sitting on the water near the center of the lake. As we approached to approximately 100 m, the gulls lifted off when an adult Bald Eagle flew toward them at a height of about 75 m. The eagle immediately started to chase a first-winter individual. The chase was linear at first, but the gull evaded the faster flying eagle with sharp turns. Over the next three minutes, the eagle continued to chase the gull with rather slow stoops from heights of 25-100 m followed by short, fast linear pursuits. -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

Chromosome Painting in Three Species of Buteoninae: a Cytogenetic Signature Reinforces the Monophyly of South American Species
Chromosome Painting in Three Species of Buteoninae: A Cytogenetic Signature Reinforces the Monophyly of South American Species Edivaldo Herculano C. de Oliveira1,2,3*, Marcella Mergulha˜o Tagliarini4, Michelly S. dos Santos5, Patricia C. M. O’Brien3, Malcolm A. Ferguson-Smith3 1 Laborato´rio de Cultura de Tecidos e Citogene´tica, SAMAM, Instituto Evandro Chagas, Ananindeua, PA, Brazil, 2 Faculdade de Cieˆncias Exatas e Naturais, ICEN, Universidade Federal do Para´, Bele´m, PA, Brazil, 3 Cambridge Resource Centre for Comparative Genomics, Cambridge, United Kingdom, 4 Programa de Po´s Graduac¸a˜oem Neurocieˆncias e Biologia Celular, ICB, Universidade Federal do Para´, Bele´m, PA, Brazil, 5 PIBIC – Universidade Federal do Para´, Bele´m, PA, Brazil Abstract Buteoninae (Falconiformes, Accipitridae) consist of the widely distributed genus Buteo, and several closely related species in a group called ‘‘sub-buteonine hawks’’, such as Buteogallus, Parabuteo, Asturina, Leucopternis and Busarellus, with unsolved phylogenetic relationships. Diploid number ranges between 2n = 66 and 2n = 68. Only one species, L. albicollis had its karyotype analyzed by molecular cytogenetics. The aim of this study was to present chromosomal analysis of three species of Buteoninae: Rupornis magnirostris, Asturina nitida and Buteogallus meridionallis using fluorescence in situ hybridization (FISH) experiments with telomeric and rDNA probes, as well as whole chromosome probes derived from Gallus gallus and Leucopternis albicollis. The three species analyzed herein showed similar karyotypes, with 2n = 68. Telomeric probes showed some interstitial telomeric sequences, which could be resulted by fusion processes occurred in the chromosomal evolution of the group, including the one found in the tassociation GGA1p/GGA6. -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -

RECENT LITERATURE Reviews by Donald S
Vo,.1944xv RecentLiterature [161 raisedand the parents,one or both,were observedmany times carryingfood to the young. In October I securedthe nest for close examinationand still have it. This is the firsttime in the yearsthe HouseWrens have lived on my placethat they have usedthe nest of any other bird or, in fact, built in any but one of my nesting boxes.-•LAUREI•CEB. FLETCHER,Cohasset, Massachusetts. Eastern Goldfinch Makes an 800-Mile Trip.-•A male Goldfinch banded at Ardmore, Pa., by Horace Groskin on April 6, 1942, •vas found dead July, 1943, near St. Andrews,New Brunswick.---HoRacEGRoss:•, 210 Glenn Road, Ardmore, Pennsylvania. RECENT LITERATURE Reviews by Donald S. Farner BANDING 1. Migration of the Red,head from the Utah Breeding Grounds. Cecil Williams. 1944. The Auk, 61(2): 251-259. Of 2,332 young Redheads(Nyroca americana (Eyton)), banded in northern Utah in 1929, 1930 and 1931 there were 357 returns, all shot by sportsmen. The northward dispersionin fall is well illus- trated by September and October returns from Montana, Wyoming, North Dakota, South Dakota and Idaho. Although migration may begin early, many young remain in the breeding grounds until October. The returns show the principal wintering grounds for the Utah birds to be the Salton Sea region of southernCalifornia and the lower coast of Texas from Corpus Christi to Mexico. Eighty-sevenpercent of the birds taken were less than one year old; 10.6 percent were second-yearbirds, and two percent were third year or older. This ratio prompts the author to assumelogically that the first year is the critical one in the life of the bird insofar as shooting is concerned. -

Olfactory Sensitivity of the Turkey Vulture (Cathartes Aura) to Three Carrion-Associated Odorants
OLFACTORY SENSITIVITY OF THE TURKEY VULTURE (CATHARTES AURA) TO THREE CARRION-ASSOCIATED ODORANTS STEVEN A. SMITH • AND RICHARD A. PASELK Departmentsof BiologicalSciences and Chemistry, Humboldt State University, Arcata,California 95523 USA ABSTRACT.--TheTurkey Vulture (Cathartesaura) is generally thought to rely on olfactory cuesto locate carrion. Becausevertically rising odorantsare dispersedrapidly by wind tur- bulence, we predict that Turkey Vultures should be highly sensitive to these chemicalsto detect them at foraging altitudes. Olfactory thresholdsto three by-productsof animal decomposition(1 x 10-6 M for buta- noic acid and ethanethiol, and 1 x 10-5 M for trimethylamine) were determined from heart- rate responses.These relatively high thresholds indicate that these odorantsare probably not cuesfor foraging Turkey Vultures. Odorant thresholds,food habits of Turkey Vultures, and the theoretical properties of odorant dispersion cast some doubt on the general impor- tanceof olfaction in food locationby this species.Received 23 September1985, accepted 3 March 1986. THEsensory modality by which Turkey Vul- Companydiscovered that natural gas leaks could tures (Cathartes aura) locate carrion has been be tracedby injectingethanethiol into gaslines debatedby naturalistsfor nearly 140 years(see and patrolling the lines for Turkey Vultures Stager1964 for review). Most of the controver- that, ostensibly,were attractedto the metcap- sy concernedwhether olfaction or vision was tan (Stager 1964). Stager (1964: 56) concluded the more important sense,although other the- from anatomical examinations and field tests ories included an "occult" sense (Beck 1920), that the Turkey Vulture "possessesand utilizes the noiseof carrion-eatingrodents, or the noise a well developedolfactory food locatingmech- of carrion-eatinginsects (Taber 1928, Darling- anism." ton 1930) as attractingTurkey Vultures to their If Turkey Vulturesrely on olfactorycues to prey. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Uncorrected Proof
Policy and Practice UNCORRECTED PROOF BBarney_C011.inddarney_C011.indd 117979 99/13/2008/13/2008 44:11:24:11:24 PPMM UNCORRECTED PROOF BBarney_C011.inddarney_C011.indd 118080 99/13/2008/13/2008 44:11:24:11:24 PPMM Copy edited by Richard Beatty 11 Conservation Values from Falconry Robert E. Kenward Anatrack Ltd and IUCN-SSC European Sustainable Use Specialist Group, Wareham, UK Introduction Falconry is a type of recreational hunting. This chapter considers the conser- vation issues surrounding this practice. It provides a historical background and then discusses how falconry’s role in conservation has developed and how it could grow in the future. Falconry, as defi ned by the International Association for Falconry and Conservation of Birds of Prey (IAF), is the hunting art of taking quarry in its natural state and habitat with birds of prey. Species commonly used for hunt- ing include eagles of the genera Aquila and Hieraëtus, other ‘broad-winged’ members of the Accipitrinae including the more aggressive buzzards and their relatives, ‘short-winged’ hawks of the genus Accipiter and ‘long-winged’ falcons (genus Falco). Falconers occur in more than 60 countries worldwide, mostly in North America, the Middle East, Europe, Central Asia, Japan and southern Africa. Of these countries, 48 are members of the IAF. In the European Union falconry Recreational Hunting, Conservation and Rural Livelihoods: Science and Practice, 1st edition. Edited by B. Dickson, J. Hutton and B. Adams. © 2009 Blackwell Publishing, UNCORRECTEDISBN 978-1-4051-6785-7 (pb) and 978-1-4051-9142-5 (hb). PROOF BBarney_C011.inddarney_C011.indd 118181 99/13/2008/13/2008 44:11:24:11:24 PPMM Copy edited by Richard Beatty 182 ROBERT E. -
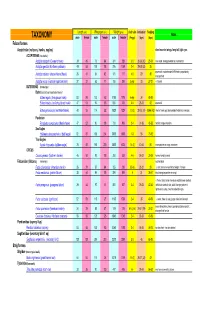
Avian Taxonomy
Length (cm) Wing span (cm) Weight (gms) cluch size incubation fledging Notes TAXONOMY male female male female male female (# eggs) (days) (days) Falconiformes Accipitridae (vultures, hawks, eagles) short rounded wings; long tail; light eyes ACCIPITRINAE (true hawks) Accipiter cooperii (Cooper's hawk) 39 45 73 84 341 528 3-5 30-36 (30) 25-34 crow sized; strongly banded tail; rounded tail Accipiter gentilis (Northern goshawk) 49 58 101 108 816 1059 2-4 28-38 (33) 35 square tail; most abundant NAM hawk; proportionaly 26 31 54 62 101 177 4-5 29 30 Accipiter striatus (sharp-shinned hawk) strongest foot Accipiter nisus (Eurasian sparrowhawk ) 37 37 62 77 150 290 5-Apr 33 27-31 Musket BUTEONINAE (broadwings) Buteo (buzzards or broad tailed hawks) Buteo regalis (ferruginous hawk) 53 59 132 143 1180 1578 6-Apr 34 45-50 Buteo lineatus (red-shouldered hawk) 47 53 96 105 550 700 3-4 28-33 42 square tail Buteo jamaicensis (red-tailed hawk) 48 55 114 122 1028 1224 1-3 (3) 28-35 (34) 42-46 (42) (Harlan' hawk spp); dark patagial featehres: immature; Parabuteo Parabuteo cuncincutus (Harris hawk) 47 52 90 108 710 890 2-4 31-36 45-50 reddish orange shoulders Sea Eagles Haliaeetus leucocephalus (bald eagle) 82 87 185 244 3000 6300 1-3 35 70-92 True Eagles Aquila chrysaetos (golden eagle) 78 82 185 220 3000 6125 1-4 (2) 40-45 50 white patches on wings: immature; CIRCUS Circus cyaneus (Northern harrier) 46 50 93 108 350 530 4-6 26-32 30-35 hovers; hunts by sound Falconidae (falcons) (longwings) notched beak Falco columbarius (American merlin) 26 29 57 64 -

Biodiversity Observations
Biodiversity Observations http://bo.adu.org.za An electronic journal published by the Animal Demography Unit at the University of Cape Town The scope of Biodiversity Observations consists of papers describing observations about biodiversity in general, including animals, plants, algae and fungi. This includes observations of behaviour, breeding and flowering patterns, distributions and range extensions, foraging, food, movement, measurements, habitat and colouration/plumage variations. Biotic interactions such as pollination, fruit dispersal, herbivory and predation fall within the scope, as well as the use of indigenous and exotic species by humans. Observations of naturalised plants and animals will also be considered. Biodiversity Observations will also publish a variety of other interesting or relevant biodiversity material: reports of projects and conferences, annotated checklists for a site or region, specialist bibliographies, book reviews and any other appropriate material. Further details and guidelines to authors are on this website. Lead Editor: Arnold van der Westhuizen – Paper Editor: Amour McCarthy and Les G Underhill INTERNET SEARCHING OF BIRD–BIRD ASSOCIATIONS: A CASE OF BEE-EATERS HITCHHIKING LARGE AFRICAN BIRDS Peter Mikula & Piotr Tryjanowski Recommended citation format: Mikula P, Tryjanowski P. 2016. Internet searching of bird–bird associations: A case of bee-eaters hitchhiking large African birds. Biodiversity Observations 7.80: 1–6. URL: http://bo.adu.org.za/content.php?id=273 Published online: 17 November 2016 – -

Are Insular Populations of the Philippine Falconet (Microhierax Erythrogenys) Steps in a Cline?
The Condor 115(3):576–583 The Cooper Ornithological Society 2013 ARE INSULAR POPULATIONS OF THE PHILIPPINE FALCONET (MICROHIERAX ERYTHROGENYS) STEPS IN A CLINE? TODD E. KATZNER1,2,5 AND NIGEL J. COLLAR3,4 1Division of Forestry and Natural Resources, West Virginia University, Morgantown, WV 26506-6125 2USDA Forest Service, Timber and Watershed Laboratory, Parsons, WV 3BirdLife International, Cambridge CB3 0NA, UK 4Natural History Museum, Tring, Herts HP23 6AP, UK Abstract. Founder effects, new environments, and competition often produce changes in species colonizing islands, although the resulting endemism sometimes requires molecular identification. One method to identify fruitful areas for more detailed genetic study is through comparative morphological analyses. We measured 210 museum specimens to evaluate the potential morphological consequences of colonization across the Philippine archipelago by the Philippine Falconet (Microhierax erythrogenys). Measurements of both males and females dif- fered clearly from island to island. Univariate and multivariate analysis of characteristics showed a latitudinal gra- dient, with the bill, wing, and tail of southern birds being larger than those of northern birds, forming the pattern of a stepped cline across a succession of islands. The stepped gradient in morphology and extensive differences between islands we observed provide evidence for multiple perspectives on phylogeny, including concordance with aggregate complexes expected on the basis of sea-level fluctuations. However, calculation of diagnosability indices did not support subspecific designations. Sex-specific dominance and dispersal patterns may explain this unusual south-to-north stepped cline, and they also provide a useful format for understanding biogeographical patterns by island. Finally, these morphological data suggest a potentially fruitful area for future genetic studies.