3D/2D Bi2s3/Sns2 Heterostructures: Superior Charge Separation And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dr. Poulami Saha
Dr. Poulami Saha Nationality: Indian (+91) 8016082561 Date of birth: 16/12/1984 Gender: Female Email address: [email protected] Address : Department of Zoology, Balurghat College Dakshin Dinajpur. West Bengal, 733101 Balurghat (India) WORK EXPERIENCE State Aided College Teacher [ 15/11/2008 – Current ] City: Balurghat Country: India ◦ Teaching area : Cell Biology, Ecology, Developmental Biology, Endocrinology and Reproductive Biology, Physiology, Evolution, Taxonomy. ◦ Head of the Department from 22.05.2009 to 25.04.2010. ◦ Appointed as External and Internal examiner for B.Sc. part I, part II, part III zoology General theory and practical examinations from 2009 ◦ Appointed as internal examiner for B.Sc. part II zoology Honors examinations (2014 - 2018) ◦ Appointed as question paper setter for zoology ◦ Appointed as External examiner (2014-2015) for Home-science Researcher [ 15/09/2012 – 21/01/2019 ] City: The University of Burdwan Country: India ◦ Collection and rearing of dipteran insects (ceratopogonids specially) ◦ Mounting of insects ◦ Studied their biosystematics EDUCATION AND TRAINING Ph. D. in Zoology ( Entomology ) The University of Burdwan [ 2014 – 2019 ] Address: Burdwan, M. Sc. in Zoology University of North Bengal [ 2006 – 2008 ] Address: Darjeeling 1 / 4 B. Ed. University of Gour Banga [ 2010 – 2011 ] LANGUAGE SKILLS Mother tongue(s): Bengali English Hindi LISTENING: B2 READING: B2 WRITING: B2 LISTENING: C1 READING: A2 SPOKEN PRODUCTION: B2 SPOKEN INTERACTION: B2 SPOKEN PRODUCTION: C1 SPOKEN INTERACTION: C1 DIGITAL SKILLS Can work with Microsoft Office PUBLICATIONS Research Publications ◦ Brahma S, Saha P, Hazra N, 2016, Two new species and new records of biting midges of the genus Dasyhelea Kieffer (Diptera: Ceratopogonidae) from India. Annales de la Société entomologique de France (N.S.), Vol. -

Department of Geography & IQAC, Gazole Mahavidyalaya
B R O C H U R E GAZOLE MAHAVIDYALAYA P.O & P.S.- Gazole, Dist.- Malda, WB. Pin-732124 www.gazolemahavidyalaya.org ONE DAY INTERNATIONAL SEMINAR ON CONTEMPORARY ENVIRONMENTAL ISSUES AND CHALLENGES FROM MULTIDISCIPLINARY STANDPOINT: SUSTAINABLE DEVELOPMENT AT THE CROSSROADS Organized by Department of Geography & IQAC, Gazole Mahavidyalaya Chair Person Patron-in-Chief Dr. Md. Shamsul Haque Sri Suresh Ch. Rano Principal, Administrator Gazole Mahavidyalaya. Gazole Mahavidyalaya Malda, WB & SDO, Malda Sadar, WB Sub themes: About the college: ■ Physical processes and Geo-environmental issues Gazole Mahavidyalaya, affiliated to University of Gour Banga, was established in the year of 2006. It is a ■ Climate Change and environmental issues and SDG Government-aided Degree College, enlisted under section 2(f) ■ Changing Interaction of man and his environment and the & 12(B) of the UGC Act, 1956. The college is situated in a resultant human and socio-economic patterns Scheduled Caste and Scheduled Tribe dominated rural area ■ Development & Sustainability: Relevance, Issues and with a vision to provide excellent educational opportunities Challenges that help the students to meet economic, social and ■ Application of RS & GIS in monitoring environmental hazard environmental challenges. Presently the college offers several under-graduate courses under the faculty of Arts and Faculty and disaster of Science. The college is situated in the Northern part of the ■ Multidisciplinary Approaches: Recent trends and Relevance district and about 20 km away from the Malda town in West in environmental research Bengal. About the Theme: The history of human civilization has had been very much impregnated with the history of environmental exploitation. With the advancement of time human beings modified the environment to suit them better. -

Balurghat College Mathematics Camp (21St October to 10Th November, 2018) Balurghat College, Balurghat Dakshin Dinajpur, West Bengal, India
Balurghat College Mathematics Camp (21st October to 10th November, 2018) Balurghat College, Balurghat Dakshin Dinajpur, West Bengal, India About the Department of Mathematics Contact Information Advisory Committee Registration Details The Department of Mathematics started functioning in 1956 with a few sincere All correspondence relating to the Camp may be communicated to the email: • Sri. Ajay Kumar Saha, President, Governing body, Balurghat College and enthusiastic teachers. Today it consists of three energetic teachers who The registration will be start on 06.09.2018 and it will be ended on [email protected] or over Mobile No.: 09775550173 (Dr. Laxman • Dr. Pankaj Kundu, Principal, Balurghat College have been doing their level best to serve the students and also they are engaged 26.09.2018. Registration fees is to be paid by electronic fund transfer in Saha, Coordinator of BCMC). • Prof. Kalishankar Tiwary, Professor, Raiganj University. in active research work. Teachers are also supervising research students to ob- favour of “Balurghat College Mathematics Camp", payable at In- • Dr. Avishek Adhikari, Associate Professor (Coordinator of NBHM in West tain their PhD degrees. Teachers are involved in various Research Projects for dian Overseas Bank, Balurghat Branch, Dakshin Dinajpur, West Bengal Zone), Calcutta University. carrying out research work. Course Details Bengal, Account Name: Balurghat College General Fund, Account No: 324501000000551, IFSC Code: IOBA0003245, MICR Code: Objective and Theme of the Camp Real Analysis: Interior points, limit points, open sets, closed sets, bounded Course Coordinator 733020102. sets, connected sets, compact sets, completeness of R. Sequences and Series of Registration Fees Structure The focus of this program is on problem solving, exploration of mathematical Real Numbers, Limit, continuity, uniform continuity, lim-sup, lim-inf, pointwise Dr. -

Gourbanga University
GOURBANGA UNIVERSITY COLLEGE SUBJECT GENERAL OBC(A) OBC(B) PH/VH SC ST TOTAL BALURGHAT COLLEGE LIBRARIAN 1 1 BENGALI 1 1 BOTANY 1 1 CHEMISTRY 2 1 1 4 COMMERCE 3 1 4 ECONOMICS 1 1 2 GEOGRAPHY 1 1 HISTORY 1 1 2 MATHEMATICS 1 1 PHILOSOPHY 1 1 PHYSICS 2 1 3 SANSKRIT 2 1 3 ZOOLOGY 1 1 2 BALURGHAT MAHILA MAHAVIDYALAYA LIBRARIAN 1 1 BENGALI 1 1 BOTANY 1 1 CHEMISTRY 1 1 ENGLISH 1 1 PHILOSOPHY 1 1 2 POLITICAL SCIENCE 1 1 2 SANSKRIT 1 1 ZOOLOGY 1 1 BUNIYADPUR MAHAVIDYALAYA ENGLISH 1 1 HISTORY 1 1 POLITICAL SCIENCE 1 1 CHANCHAL COLLEGE BENGALI 1 1 ECONOMICS 1 1 ENGLISH 1 1 HISTORY 1 1 MATHEMATICS 1 1 PHILOSOPHY 1 1 SANSKRIT 2 2 DEWAN ABDUL GANI COLLEGE EDUCATION 1 1 ENGLISH 1 1 PHILOSOPHY 1 1 POLITICAL SCIENCE 1 1 SANSKRIT 1 1 DR. MEGHNATH SAHA COLLEGE LIBRARIAN 1 1 EDUCATION 1 1 ENGLISH 1 1 HISTORY 1 1 MATHEMATICS 1 1 PHYSICS 1 1 POLITICAL SCIENCE 1 1 SANSKRIT 1 1 SOCIOLOGY 1 1 GAJOLE MAHAVIDYALAYA BENGALI 1 1 EDUCATION 1 1 ENGLISH 1 1 GEOGRAPHY 1 1 HISTORY 1 1 POLITICAL SCIENCE 1 1 2 SOCIOLOGY 1 1 GANGARAMPUR COLLEGE LIBRARIAN 1 1 BENGALI 1 1 CHEMISTRY 1 1 EDUCATION 1 1 GEOGRAPHY 1 1 POLITICAL SCIENCE 1 1 SANSKRIT 1 1 ZOOLOGY 1 1 GOUR MAHAVIDYALAYA COMPUTER SCIENCE 1 1 2 EDUCATION 1 1 ENGLISH 1 1 GEOGRAPHY 1 1 PHYSICS 1 1 POLITICAL SCIENCE 1 1 2 SOCIOLOGY 1 1 ZOOLOGY 1 1 JAMINI MAZUMDAR MAMORIAL COLLEGE BENGALI 1 1 PHILOSOPHY 1 1 POLITICAL SCIENCE 1 1 KALIACHAK COLLEGE EDUCATION 1 1 ENGLISH 1 1 2 KALIAGANJ COLLEGE LIBRARIAN 1 1 BENGALI 1 1 COMMERCE 1 1 2 ECONOMICS 1 1 ENGLISH 1 1 1 3 HINDI 1 1 HISTORY 1 1 MATHEMATICS 1 2 3 PHILOSOPHY 1 1 PHYSICS -

Provisional Result of UG Part I (General) Supplementary Examination 2019 College Roll No
University of Gour Banga (Established under the West Bengal Act. XXVI of 2007) [Recognized U/S 2(f) & 12(B) of the UGC Act and NAAC accredited University with ‘B’ Grade (2016)] Provisional Result of UG Part I (General) Supplementary Examination 2019 College Roll No. Regn. No. Session Stream Remark Back Subject Balurghat College 0216GENA 1073 0211473 2015-2016 B.A. Q-II Balurghat College 0217GENA 0151 0210164 2016-2017 B.A. Q-II Balurghat College 0217GENA 0216 0210228 2016-2017 B.A. Q-II Balurghat College 0217GENA 0276 0211153 2016-2017 B.A. Q-II Balurghat College 0217GENS 0022 0211788 2016-2017 B.SC. Q-II Balurghat College 0218GENA 0074 0210087 2017-2018 B.A. Q-II Balurghat College 0218GENA 0107 0210128 2017-2018 B.A. Q-II Balurghat College 0218GENA 0187 0210218 2017-2018 B.A. Q-II Balurghat College 0218GENA 0233 0210273 2017-2018 B.A. Q-II Balurghat College 0218GENA 0393 0210451 2017-2018 B.A. Q-II Balurghat College 0218GENA 0478 0211266 2017-2018 B.A. Q-II Balurghat College 0218GENA 0555 0211135 2017-2018 B.A. Q-II Balurghat College 0218GENA 0668 0210734 2017-2018 B.A. Q-II Balurghat College 0218GENA 0801 0210864 2017-2018 B.A. Q-II Balurghat College 0218GENA 0833 0211149 2017-2018 B.A. Q-II Balurghat College 0218GENA 0887 0210952 2017-2018 B.A. Q-II Balurghat College 0218GENA 0904 0210968 2017-2018 B.A. Q-II Balurghat College 0218GENA 0913 0210977 2017-2018 B.A. Q-II Balurghat College 0218GENA 0935 0211005 2017-2018 B.A. Q-II Balurghat College 0218GENA 0984 0211060 2017-2018 B.A. -

One Day National Webinar Sustainable Development
Raiganj Surendranath Mahavidyalaya NAAC accredited College with “B+” Grade (December’ 2016) Department of Geography Raiganj, Uttar Dinajpur, West Bengal, INDIA, PIN 733134 www.rsmraiganj.in One Day National Webinar on Sustainable Development: Target and Achievement 29 August 2020 (Please join us at 9:45 AM on 29 August; Registration is free) Joining Subject Timing People Registration Link https://forms.gle/eyPpYsN81v8GMrP87 WhatsApp Group https://chat.whatsapp.com/Jxco18Q1Se27NtOUBB1XhX Google Meet Link https://meet.google.com/iit-becb-cvn For any Query 9735069222 [email protected] Inaugural Session Welcome Address 10.00 AM Dr. Prithwiraj Jha to TIC & HoD (Acting) of Dept. of Geography; 10.05 AM Assistant Professor, Department of Zoology, Raiganj Surendranath Mahavidyalaya Message of Convener 10.05 AM Tarik Anowar to Dept. of Geography 10.10 AM Raiganj Surendranath Mahavidyalaya Message of Chief Guest 10.10 AM Dr. Krishnendu Gupta to Assistant Professor, Dept. of Geography, Visva-Bharati 10.40 AM Executive Guest 10.40 AM Dr. Kanha R. Godha to Lead Urban Planner, PMU Pradhan Mantri Awash Yojna Urban, 11.10 AM Ministry of Urban Housing and Urban Affairs, Govt. of India Key Note Address 11.10 AM Prof. Dr. Piyal Basu Roy to Prof., Department of Geography, 11.40 AM Coochbehar Panchanan Barma University Special Guest 11.40 AM Advocate Bipad Taran Bhatacharjee to Advocate, Kolkata High Court 12.10 PM Session: 1 (12.10 PM to 2.10 PM) Chairperson- Dr. Prohlad Roy, Assistant Professor, Department of Education, Visva-Bharati Co- Chairperson- Dr. Md. Ismail, HoD & Assistant Professor, Dept. of Geography, Dewan Abdul Gani College & Dr. -

University of Gour Banga
University of Gour Banga District: MALDA Sl. Name of the College & Phone No. Email- id No. Address Chanchal College [1969] 1. P.O Chanchal 03513-253261 [email protected] Malda – 732 123. Gazole Mahavidyalaya 2. [2005] 03512-234264 [email protected] P.O Gazole, Malda – 732 124 Gour Mahavidyalaya [1985] 3. [email protected] P.O Mangalbari 03512-260547 [email protected] Malda – 732 141. Harishchandrapur College 4. [2008] harishchandrapurcollege2008@g 9434372031 P.O. Pipla mail.com Malda– 731 125. Kaliachak College [1995] 5. 03512- P.O Sultanganj, Kaliachak, [email protected] 244696/245309 Malda– 732 201. Malda College [1944] 6. 03512- [email protected] P.O. & Dist. Malda – 732 220807/220808 [email protected] 101. Malda Women’s College 7. [1970] 03512-252597 [email protected] P.O. & Dist. Malda- 732 101. Manikchak College 8. P.O. Mathurapur 03513 283048 [email protected] Malda– 732 203. Pakuahat Degree College 9. [1997] 03511-266410 [email protected] P.O. Pakuahat Malda – 732 138 10. Samsi College [1968] [email protected] P.O. Samsi, [email protected] 03513-265252 Malda – 732 139. [email protected] [email protected] 11. South Malda College [1995] principalsouthmaldacollege@gmai P.O. Pubarun Fax: 03512-224292 l.com Malda – 732 215. Dist.: UTTAR DINAJPUR Sl. Name of the College & Phone No. Email- id No. Address Dr. Meghnath Saha College 1. [2000] 03523-277707 [email protected] P.O. Tilna, Ranipur– 733 128. Kaliyaganj College [1968] 2. 03523-258030/ P.O. Kaliyaganj – 733 129. [email protected] 258100/259659 Raiganj Surendranath (033) 2560-2130 3. -
Highlights of Departmental Activities (Zoology Dept.) During 2018
Highlights of Departmental Activities (Zoology Dept.) during 2018 * Study tour (third year students of the 2017-18 session) Dr. Prithwiraj Jha, HoD accompanied the team to Gopalpur beach (Odhisha) and Visakhapatnam (Andhra Pradesh) for an 8- day study trip during 31 December’2017 to 08 January’2018. Students of the third year (2017-18 session) participated in the trip. Figures: The RSM team in action in Gopalpur beach. * First departmental reunion It was the first reunion of the Department of Zoology of Raiganj Surendranath Mahavidyalaya. In fact, it was the first reunion of any Department of the College. On 26 January 2018, 25 ex-students of the Department, along with students from the current batches (total gathering: 76 students) witnessed a memorable event. The inaugural session was chaired by Hon’ble TIC, Sri Arup Sanyal, which was followed by student interactions and a picnic. Figures: A colourful collage of the first reunion (Zoology Dept., RSM). * Study tour (first year students of the 2017-18 session) Dr. Prithwiraj Jha, HoD accompanied the team to Nainital and surrounding areas (Uttarakhand) for an 8- day study trip during 16 to 24 February’2018. Students of the first year (2017-18 session) participated in the trip. The students also got a rare opportunity to visit the Zoology Department (and various laboratories of the Dept.) of Kumaun University. Figure: The RSM team in Nainital. Figure: Dr. P. Jha (left photo) and students of RSM (right photo) along with Prof. Dr. B.R. Kaushal of the Zoology Dept., Kumaun University. Prof. Kaushal had earlier visited the Raiganj Surendranath Mahavidyalaya in November’2016, and received the students very warmly in his Department. -

Honours) Supplementary Examination 2019 College Roll No
University of Gour Banga (Established under the West Bengal Act. XXVI of 2007) [Recognized U/S 2(f) & 12(B) of the UGC Act and NAAC accredited University with ‘B’ Grade (2016)] Provisional Result of UG Part II (Honours) Supplementary Examination 2019 College Roll No. Regn. No. Session Stream Remark Back Subject Balurghat College 0216CMSH 0003 0212167 2015-2016 B.SC. Q-III Balurghat College 0216EDCH 0020 0211619 2015-2016 B.A. Q-III Balurghat College 0216EDCH 0024 0211625 2015-2016 B.A. Q-III Balurghat College 0216HISH 0090 0210777 2015-2016 B.A. Q-III Balurghat College 0216PHIH 0007 0211911 2015-2016 B.A. Q-III Balurghat College 0216SANH 0027 0211995 2015-2016 B.A. Q-III Balurghat College 0216SANH 0030 0212000 2015-2016 B.A. Q-III Balurghat College 0216SANH 0067 0212040 2015-2016 B.A. Q-III Balurghat College 0216SANH 0094 0212067 2015-2016 B.A. Q-III Balurghat College 0217BNGH 0015 0211254 2016-2017 B.A. Q-III Balurghat College 0217EDCH 0056 0211408 2016-2017 B.A. Q-III Balurghat College 0217ENGH 0007 0211432 2016-2017 B.A. Q-III Balurghat College 0217MTMH 0007 0211892 2016-2017 B.SC. Q-III Balurghat College 0217MTMH 0020 0211906 2016-2017 B.SC. Q-III Balurghat College 0217PHIH 0001 0211662 2016-2017 B.A. Q-III Balurghat College 0217PHIH 0023 0211682 2016-2017 B.A. Q-III Balurghat College 0218EDCH 0022 0211418 2017-2018 B.A. Q-III Balurghat College 0218MTMH 0018 0211944 2017-2018 B.SC. Q-III Balurghat College 0218PHIH 0021 0211705 2017-2018 B.A. Q-III Balurghat College 0218SANH 0040 0211772 2017-2018 B.A. -
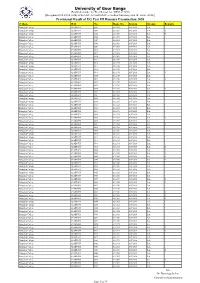
Provisional Result of UG Part III Honours Examination 2020 College Roll No
University of Gour Banga (Established under the West Bengal Act. XXVI of 2007) [Recognized U/S 2(f) & 12(B) of the UGC Act and NAAC accredited University with ‘B’ Grade (2016)] Provisional Result of UG Part III Honours Examination 2020 College Roll No. Regn. No. Session Stream Remark Balurghat College 0218BNGH 0001 0211294 2017-2018 B.A. I Balurghat College 0218BNGH 0004 0211297 2017-2018 B.A. II Balurghat College 0218BNGH 0005 0211298 2017-2018 B.A. II Balurghat College 0218BNGH 0006 0211299 2017-2018 B.A. I Balurghat College 0218BNGH 0007 0211301 2017-2018 B.A. I Balurghat College 0218BNGH 0008 0211302 2017-2018 B.A. I Balurghat College 0218BNGH 0009 0211304 2017-2018 B.A. I Balurghat College 0218BNGH 0010 0211305 2017-2018 B.A. I Balurghat College 0218BNGH 0011 0211306 2017-2018 B.A. I Balurghat College 0218BNGH 0014 0211309 2017-2018 B.A. I Balurghat College 0218BNGH 0015 0211310 2017-2018 B.A. I Balurghat College 0218BNGH 0016 0211311 2017-2018 B.A. I Balurghat College 0218BNGH 0017 0211312 2017-2018 B.A. I Balurghat College 0218BNGH 0018 0211313 2017-2018 B.A. I Balurghat College 0218BNGH 0019 0211314 2017-2018 B.A. II Balurghat College 0218BNGH 0020 0211315 2017-2018 B.A. I Balurghat College 0218BNGH 0021 0211316 2017-2018 B.A. II Balurghat College 0218BNGH 0022 0211317 2017-2018 B.A. I Balurghat College 0218BNGH 0023 0211319 2017-2018 B.A. I Balurghat College 0218BNGH 0024 0211320 2017-2018 B.A. II Balurghat College 0218BNGH 0025 0211321 2017-2018 B.A. I Balurghat College 0218BNGH 0027 0211323 2017-2018 B.A. -

Sub-Divisional Magistrate Balurghat Sadar Page I Of2 I I I Memo No
In the Court of the Sub-Divisional Magistrate, Balurghat, Dakshin Dinajpur. Prohibitory Order uls 144 (2) of Cr. P. C. West Bengal Form No. 3800 H (Criminal Form No. (M) 2) ORDER SHEET FOR MAGISTRATES' RECORDS No.M'.R. /2021. Office note as to action 81, No, taken on Order with signature of the Magistrate of Date order (if Order anl') and date Whereas, the Common Entrance Test "ANM (R) & GNM-2021" for admission in 21-08- Auxiliary Nursing & Midwifery and General Nursing & Midwifery courses under the West 2021 Bengal Joint Entrance Examinations Board will be held on 22.08.2021 in the following Centers / Venues under Balurghat Sub-Division. SI. No. Exam. Centre Police Station 1 Balurghat College Balurghat 2 Balurghat B. Ed College Balurghat 3 Bahicha L.K. High School (HS) Patiram 4 Patiram High School Patiram And, Whereas it is necessary to maintain peace and public order at the aforesaid Examination Centers / Venues during the aforesaid dates for smooth conduct of the examination, and Whereas there is apprehension of breach of peace and public order in and around the above venues, and as such it is necessary to promulgate prohibitory order to restrain these activities, Now, therefore, I, Shri Suman Dasgupta, W.B.C.S (Exe.), Sub-Divisional Magistrate, Balurghat Sadar do hereby promulgate this Order u/s 144(2) Cr. P.C, to direct that- I) The said prohibited order will remain in force in & around the above mentioned venues on,22-08-2021 (rom 10:00 A.M. to 04:30 P.M. II) No one will be allowed to move around witlrin the radius of 100 meters of the premises of the aforesaid Examination Centers / Venues with arms, weapons or anything that may cause panic among the people and the examinees. -
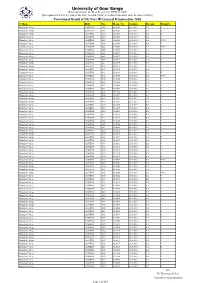
Provisional Result of UG Part III General Examination 2020 College Roll No
University of Gour Banga (Established under the West Bengal Act. XXVI of 2007) [Recognized U/S 2(f) & 12(B) of the UGC Act and NAAC accredited University with ‘B’ Grade (2016)] Provisional Result of UG Part III General Examination 2020 College Roll No. Regn. No. Session Stream Remark Balurghat College 0216GENA 0005 0210023 2015-2016 B.A. P Balurghat College 0216GENA 0008 0210024 2015-2016 B.A. II Balurghat College 0216GENA 0012 0210028 2015-2016 B.A. II Balurghat College 0216GENA 0024 0210039 2015-2016 B.A. P Balurghat College 0216GENA 0029 0210045 2015-2016 B.A. PNC2 Balurghat College 0216GENA 0034 0210049 2015-2016 B.A. P Balurghat College 0216GENA 0035 0210050 2015-2016 B.A. PNC2 Balurghat College 0216GENA 0044 0210058 2015-2016 B.A. II Balurghat College 0216GENA 0062 0210071 2015-2016 B.A. P Balurghat College 0216GENA 0063 0210072 2015-2016 B.A. P Balurghat College 0216GENA 0066 0210075 2015-2016 B.A. II Balurghat College 0216GENA 0087 0210096 2015-2016 B.A. P Balurghat College 0216GENA 0103 0210113 2015-2016 B.A. P Balurghat College 0216GENA 0107 0210116 2015-2016 B.A. P Balurghat College 0216GENA 0113 0210119 2015-2016 B.A. II Balurghat College 0216GENA 0138 0210148 2015-2016 B.A. PNC2 Balurghat College 0216GENA 0140 0210150 2015-2016 B.A. P Balurghat College 0216GENA 0145 0210156 2015-2016 B.A. P Balurghat College 0216GENA 0151 0210160 2015-2016 B.A. P Balurghat College 0216GENA 0181 0210189 2015-2016 B.A. P Balurghat College 0216GENA 0183 0211282 2015-2016 B.A. P Balurghat College 0216GENA 0184 0210191 2015-2016 B.A.