Deuterium in New Zealand Rivers and Streams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Outdoor Recreation Strategy 1 2012 - 2022 Central Otago Outdoor Recreation Sstrategytrategy 2012012222 --- 2022022222
= `Éåíê~ä=lí~Öç= =lìíÇççê=oÉÅêÉ~íáçå= píê~íÉÖó= = OMNO=J=OMOO= February 2012 This is a community owned strategy developed by the Outdoor Recreation Working Party in consultation with the Central Otago Community Central Otago Outdoor Recreation Strategy 1 2012 - 2022 Central Otago Outdoor Recreation SStrategytrategy 2012012222 --- 2022022222 PAGE EXECUTIVE SUMMARY 4 IMPLEMENTATION 8 INTRODUCTION 15 Goals 15 Why have an Outdoor Recreation Strategy? 15 What Comprises Recreation? 16 What Makes a Good Experience 16 Purpose 16 Management Approaches 16 Planning 17 Importance of Outdoor Recreation 17 Central Otago – Geographically Defined 17 Barriers to Participation in Outdoor Recreation 18 Changing Perceptions of Outdoor Recreation 19 Fragmentation of Leisure Time 19 Conflict of Use 19 Changing Perceptions of Risk 19 Developing Outdoor Skills 20 Outdoor Recreation, Individuals and Communities 20 Environmental Considerations 21 Economic Considerations 21 Key Characteristics of Central Otago 21 Other Strategies 21 Regional Identity (A World of Difference) 22 Other Agencies and Groups Involved 22 Assumptions and Uncertainties 22 OVERARCHING ISSUES Human Waste Disposal 23 Rubbish 23 Dogs 23 Signs, Route Guides and Waymarking (Geographic Information) 24 Access 24 Research 25 Landowners 25 Competing Use 26 Communications 27 SPECIFIC RECREATION ACTIVITIES Notes on Tracks, Trails and Recreational Areas 28 Air Activities 29 Mountain Biking 31 Road Cycling 38 Climbing 40 Four Wheel Driving 43 Gold Panning 47 Hunting – Small Game and Big Game 49 Central -

Pollen-Based Temperature and Precipitation Records of the Past
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers Faculty of Science, Medicine and Health 2017 Pollen-based temperature and precipitation records of the past 14,600 years in northern New Zealand (37°S) and their linkages with the Southern Hemisphere atmospheric circulation Ignacio Jara Victoria University of Wellington Rewi Newnham Victoria University of Wellington Brent V. Alloway University of Wollongong Janet M. Wilmshurst Landcare Research NZ Andrew Rees Victoria University of Wellington Publication Details Jara, I. A., Newnham, R. M., Alloway, B. V., Wilmshurst, J. M. & Rees, A. B.H. (2017). Pollen-based temperature and precipitation records of the past 14,600 years in northern New Zealand (37°S) and their linkages with the Southern Hemisphere atmospheric circulation. The oH locene: a major interdisciplinary journal focusing on recent environmental change, 27 (11), 1756-1768. Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Pollen-based temperature and precipitation records of the past 14,600 years in northern New Zealand (37°S) and their linkages with the Southern Hemisphere atmospheric circulation Abstract Regional vegetation, climate history, and local water table fluctuations for the past 14,600 years are reconstructed from pollen and charcoal records of an ombrogenous peatbog in northern New Zealand (38°S). A long-term warming trend between 14,600 and 10,000 cal. yr BP is punctuated by two brief plateaux between 14,200-13,800 and 13,500-12,000 cal. yr BP. Periods of relatively drier conditions are inferred between 14,000-13,400 and 12,000-10,000 cal. -

Heading/Title Aquatic Pest Report 2019
Aquatic Pest Report 2019 Bay of Plenty Regional Council Environmental Publication 2019/03 FebruaryHeading/title 2019 PreparedSubheading by Freya Ewing and Thomas Bailey (Summer Students) 5Month Quay YearStreet P O Box 364 WhakatānePrepared by NEW ZEALAND ISSN: 1175-9372 (Print) ISSN: 1179-9471 (Online) Cover photo: Rowan Wells (NIWA) Acknowledgements We would like to thank Hamish Lass, Biosecurity Officer for his support and guidance, and Lucas MacDonald, Biosecurity Officer for his assistance and advice across all advocacy work. Our acknowledgements also go to Greg Corbett and the remainder of the Biosecurity Team at the Bay of Plenty Regional Council, we are grateful for the encouragement and support we received from all of you. We would also like to show our gratitude to Geoff Ewert for his continued commitment to the Rotorua Lakes, in particular with fixing weed cordons and the running of the boat wash station. We are also immensely grateful to Sarah Van Der Boom from Freshwater Biosecurity Partnership Program for her help with data collection. Lastly, we would like to thank Sam Beaumont and the remainder of the team at Ministry for Primary Industries, for the supplying of merchandise and collateral, signage and guidance. BAY OF PLENTY REGIONAL COUNCIL TOI MOANA 1 Executive summary Many of New Zealand’s most well-known lakes and rivers can be found in the Bay of Plenty region and are therefore known to attract significant visitor numbers annually. The preservation and protection of these waterways are what attracts so many international and domestic tourists to them. Due to the close proximity of the Bay of Plenty lakes and rivers, and their popularity, they are extremely susceptible to invasive pest species. -

Natural Character, Riverscape & Visual Amenity Assessments
Natural Character, Riverscape & Visual Amenity Assessments Clutha/Mata-Au Water Quantity Plan Change – Stage 1 Prepared for Otago Regional Council 15 October 2018 Document Quality Assurance Bibliographic reference for citation: Boffa Miskell Limited 2018. Natural Character, Riverscape & Visual Amenity Assessments: Clutha/Mata-Au Water Quantity Plan Change- Stage 1. Report prepared by Boffa Miskell Limited for Otago Regional Council. Prepared by: Bron Faulkner Senior Principal/ Landscape Architect Boffa Miskell Limited Sue McManaway Landscape Architect Landwriters Reviewed by: Yvonne Pfluger Senior Principal / Landscape Planner Boffa Miskell Limited Status: Final Revision / version: B Issue date: 15 October 2018 Use and Reliance This report has been prepared by Boffa Miskell Limited on the specific instructions of our Client. It is solely for our Client’s use for the purpose for which it is intended in accordance with the agreed scope of work. Boffa Miskell does not accept any liability or responsibility in relation to the use of this report contrary to the above, or to any person other than the Client. Any use or reliance by a third party is at that party's own risk. Where information has been supplied by the Client or obtained from other external sources, it has been assumed that it is accurate, without independent verification, unless otherwise indicated. No liability or responsibility is accepted by Boffa Miskell Limited for any errors or omissions to the extent that they arise from inaccurate information provided by the Client or -

Ïg8g - 1Gg0 ISSN 0113-2S04
MAF $outtr lsland *nanga spawning sur\feys, ïg8g - 1gg0 ISSN 0113-2s04 New Zealand tr'reshwater Fisheries Report No. 133 South Island inanga spawning surv€ys, 1988 - 1990 by M.J. Taylor A.R. Buckland* G.R. Kelly * Department of Conservation hivate Bag Hokitika Report to: Department of Conservation Freshwater Fisheries Centre MAF Fisheries Christchurch Servicing freshwater fisheries and aquaculture March L992 NEW ZEALAND F'RESTTWATER F'ISHERIES RBPORTS This report is one of a series issued by the Freshwater Fisheries Centre, MAF Fisheries. The series is issued under the following criteria: (1) Copies are issued free only to organisations which have commissioned the investigation reported on. They will be issued to other organisations on request. A schedule of reports and their costs is available from the librarian. (2) Organisations may apply to the librarian to be put on the mailing list to receive all reports as they are published. An invoice will be sent for each new publication. ., rsBN o-417-O8ffi4-7 Edited by: S.F. Davis The studies documented in this report have been funded by the Department of Conservation. MINISTBY OF AGRICULTUBE AND FISHERIES TE MANAlU AHUWHENUA AHUMOANA MAF Fisheries is the fisheries business group of the New Zealand Ministry of Agriculture and Fisheries. The name MAF Fisheries was formalised on I November 1989 and replaces MAFFish, which was established on 1 April 1987. It combines the functions of the t-ormer Fisheries Research and Fisheries Management Divisions, and the fisheries functions of the former Economics Division. T\e New Zealand Freshwater Fisheries Report series continues the New Zealand Ministry of Agriculture and Fisheries, Fisheries Environmental Report series. -

Walks in the Westport Area, West Coast
WEST COAST Look after yourself Your safety is your responsibility Walks in the Choose a walk that matches the weather and your own • Plan your trip experience, and interests you. Know what the weather • Tell someone your plans is doing – it can change dramatically in a short time. • Be aware of the weather Westport area Call at Department of Conservation (DOC) offices or Visitor Centres to check current weather and • Know your limits track conditions. • Take sufficient supplies Times given are a guide only, and will vary depending on Visit www.mountainsafety.org.nz to learn more. fitness, weather and track conditions. For walks longer than an hour, pack a small first aid kit and take extra food and drink. Insect repellent is recommended to ward off sandflies and mosquitoes. Cape Foulwind Walkway Photo: Miles Holden The combined output of coal mines and sawmills helped create a remarkable railway up the sheer-sided Ngakawau Gorge to Charming Creek. It is now used by thousands of walkers who rate it one of the best walkways around. Westport had the West Coast’s earliest gold diggings The Westport area extends from and has some of the best-preserved reminders of this the Mokihinui River in the north vibrant period. Your historical wanderings can range from the haunting hillside site of Lyell, which many to Tauranga Bay in the south, and motorists pass unaware of, to the lonely Britannia inland to the Buller Gorge, including battery, reached by determined trampers via a several mountain ranges. It is valley track. wonderfully diverse. Even the highways have historic features, including Hawks Crag, a low-roofed ledge blasted out of solid There is a great range of walking rock in the lower Buller Gorge, and the stone-piered Iron Bridge in the upper gorge. -

Downloaded 10/04/21 09:37 AM UTC SW-NE Axis for a Distance of 800 Km
The New Zealand *** Southern Alps Experiment ^ D. S. Wratt,* R. N. Ridley,* M. R. Sinclair,* H. Larsen,* S. M. Thompson,* R. Henderson/ G. L. Austin,* S. G. Bradley,* A. Auer,@ A. P. Sturman,& I. Owens,& B. Fitzharris,** B. F. Ryan,++ and J.-F. Gayet** ABSTRACT The Southern Alps Experiment is being mounted to study the influence of New Zealand's Southern Alps on local weather and climate. This paper describes these alpine influences and outlines proposed field and modeling experi- ments. Experiment goals include understanding and quantifying factors that govern the intensity and spatial distribu- tion of heavy rainfall, the west to east distribution of precipitation across the mountains, and the intensity of lee wind storms and warming. Linked research will explore the use of deterministic rainfall models to predict river flows from mountain watersheds. 1. Introduction 95), mountain influences on South Island weather have been explored using archived weather data and The Southern Alps Experiment (SALPEX) is a new mesoscale models, and two initial field campaigns study of the processes through which the Alps influ- have been mounted. Results from this first phase are ence the weather and climate of New Zealand. The being used to refine experimental plans and develop Southern Alps form a long, narrow barrier across the hypotheses for testing during the second phase, which predominantly western flow over New Zealand's will include the main field work component (1996- South Island. In the first phase of SALPEX (1993- 97). In the final phase the experimental and model- ing results will be consolidated into an improved quan- *National Institute of Water and Atmospheric Research, titative understanding of mountain influences on New Wellington, New Zealand. -

Apteryx Haastii)
Genetic variability, distribution and abundance of great spotted kiwi (Apteryx haastii) John McLennan and Tony McCann ABSTRACT Recent information on the genetic composition, morphological features, distri- bution and abundance of great spotted kiwi is collated, analysed and used to evaluate the species’ conservation status. The species exhibits high levels of genetic variability but with no consistent geographical patterns. Some morphological variability is present along latitudinal and altitudinal gradients. Further study is warranted to determine if genetically and/ or morphologically distinct populations are present. There are currently about 22 000 great spotted kiwi spread over 6000 km2 in the northwest of the South Island. Most great spotted kiwi now live in high rainfall mountainous regions. There are three principal populations: in Northwest Nelson, the Paparoa Range, and the Arthur’s Pass–Hurunui district. Since European settlement, the species has become extirpated in eastern Nelson, part of North Westland, and the central Westland mountain valleys. Overall, the species’ range has contracted by at least 30%. The principal cause of decline is probably predation by stoats (Mustela erminea). Conservation of the intra-specific genetic and morphological diversity of great spotted kiwi is best achieved by protecting the range of extant populations, although this may be logistically difficult. The focus will probably settle on the three main population groups. We expect further declines to take place, particularly in southern Northwest Nelson and in Arthur’s Pass–Hurunui. The large populations in upland areas of Northwest Nelson and the Paparoa Range are probably stable but this needs to be monitored. The species should be classified as ‘vulnerable’ according to IUCN threat definitions. -
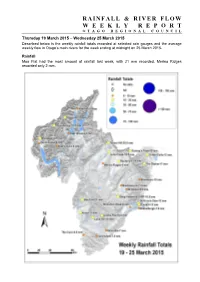
Rainfall & River Flow Weeklyreport
RAINFALL & RIVER FLOW WEEKLY REPORT OTAGO REGIONAL COUNCIL Thursday 19 March 2015 – Wednesday 25 March 2015 Described below is the weekly rainfall totals recorded at selected rain gauges and the average weekly flow in Otago’s main rivers for the week ending at midnight on 25 March 2015. Rainfall Moa Flat had the most amount of rainfall last week, with 21 mm recorded. Merino Ridges recorded only 2 mm. River Flows Flows in the Manuherikia River, Shotover River, Kawarau River, Waipahi River, and the Clutha River at Balclutha were below normal. The Kakanui River at Clifton Falls was the only flow recorder having above normal flows. Table 1. River flow information for Otago’s main rivers (all flows in cumecs, m3/s) Weekly River and Site Name Minimum Maximum State Average Kakanui River at Clifton Falls 1.391 0.919 2.680 above normal Shag River at The Grange 0.219 0.164 0.332 normal Taieri River at Canadian Flat 2.126 1.290 4.409 normal Taieri River at Tiroiti 2.728 2.084 5.377 normal Taieri River at Sutton 3.335 2.469 5.636 normal Taieri River at Outram 5.884 4.823 8.000 normal Clutha River at Balclutha 376.966 294.501 505.056 below normal Waipahi River at Waipahi 0.672 0.542 0.905 below normal Pomahaka River at Burkes Ford 9.157 7.009 12.915 normal Manuherikia River at Ophir 1.869 1.418 2.270 below normal Clutha R. at Cardrona Confluence 229.972 145.042 288.669 normal Kawarau River at Chards Rd 126.705 119.148 135.372 below normal Shotover River at Peat's Hut 10.783 10.122 13.659 below normal Lake Levels Water levels in Lake Hawea and Lake Wakatipu were both well below normal. -

Seabed Mining (KASM), Posted a Facebook Page Inviting People to Turn up at the Stadium When Hearings Began
MINING MINEFIELD A company called Trans-Tasman Resources is having a second go at setting up a world-first project to mine iron ore from the seabed off South Taranaki’s coast. The company (TTR) has spent a decade and about $70 million to hone its case for Environmental Protection Authority (EPA) consent to take more than a billion tonnes of iron- bearing sand off the sea floor over 25 or so years. The process – which will return 90 percent of what’s extracted back to the seabed - will bring in about $400 million a year in off-shore ore sales, with our government getting about $6 million in annual royalties. The project is expected to increase Taranaki’s gross domestic product by about $220 million a year (half that of Methanex) and create about 300 jobs. Sound like a good deal? Somewhere between 13,733 and about 17,000 people (the total is disputed) don’t think so. Many people living in South Taranaki and further afield, local iwi, most of the fishing industry, and a close-neighbour oil company are worried about what it might do to the environment and local communities. That uncertainty has prompted their representative organisations and many individuals to fight the company to the bitter end. The case will be a precedent-setter under the Exclusive Economic Zone (EEZ) Act. Environmentalists don’t like the law, partly because it excludes climate change-causing emissions as grounds for objection, and partly because they reckon it’s the National government’s way of opening up our 200 kilometre-wide continental shelf to big overseas business. -

Whanganui Headwaters AD.Pub
Ongarue River Waimiha Stream Te Kui Despite its relave remoteness the Ongarue is a popular river offer- A delighul stream holding large numbers of rainbows and the occa- ing a good catch rate of large rainbow trout as well as many kilome- Te Kui to Mangakino sional big brown. The lower reaches offer clear, low banks providing tres of fishing water. 30 relavely easy fishing - a perfect place for the beginner. Further up- The lower reaches below the confluence with the Waimiha are rarely stream, the Waimiha flows through scrub and nave forest providing fished, somewhat surprising as this secon offers some very good Benneydale aracve fishing waters. Some good fish are taken from this secon. spin fishing waters especially in the gorge downstream from the Access is from Waimiha Road across farmland. Although there is a sign at the gate stang otherwise the Waimaiha Valley Road is a pa- Waimiha Township. Access is from the Waimiha-Ongarue Road which Waimiha Rd runs parallel to the river. per road and there is public access to the river about 5 km from the Waimiha Stream gate. Please get permission from the farmhouse just north of the Access to the middle reaches can be gained across farmland from the Waimiha-Ongarue Road turnoff or call the number on the gate. Ongarue Stream Road, which runs parallel to the river (landowners Waimiha Valley Rd permission required). The first public access via an unformed paper In the upper reaches the Waimiha is a small stream that meanders Te Kui across farmland with stable pools and runs. -
![Proposals Considered by the NZGB on 18 October 2019 For: Ngāmotu [Assign a New Name]](https://docslib.b-cdn.net/cover/9765/proposals-considered-by-the-nzgb-on-18-october-2019-for-ng%C4%81motu-assign-a-new-name-1029765.webp)
Proposals Considered by the NZGB on 18 October 2019 For: Ngāmotu [Assign a New Name]
Proposals considered by the NZGB on 18 October 2019 for: Ngāmotu [assign a new name] Otangiwao [altered from Otangiwai Point] NZTopo50-BE38 Crown copyright reserved Inset map LINZ ‘Topo’ basemap Summary The proposals are to restore two original Māori names by: - assigning Ngāmotu to an unnamed peninsula on the west shores of Lake Rotomā, approximately 30km east-northeast of Rotorua, and - altering Otangiwai Point (unofficial recorded) to Otangiwao, for the small point on the southwest shore of Lake Rotomā, correcting the spelling and dropping the English generic term, Point. The proposers, Ngāti Tamateatūtahi/Ngāti Kawiti, advise they are mana whenua, and their preference is no English generic term be included with either name. A local marae, Tapuaeharuru, Te Tatau o Te Arawa (a governance body), and the Bay of Plenty Regional Council support the proposals. Ngāti Pikiao, Rotorua Lakes District Council, and other iwi and post Settlement entities with interests in the area have not provided their views. Ngāmotu names are duplicated many times in the North Island, including a hill ~30km southeast of the proposal. The NZGB may consider adding a generic term to Ngāmotu, for example, Ngāmotu Peninsula, to make the name unique and the feature easily identifiable in an emergency. A local use name Doctors Point may apply to the entire feature or only the eastern end. Otangiwai Point is a popular site for diving, including commercial operations, so to identify the feature in an emergency the NZGB may consider retaining the ‘Point’ generic term, which is in long term use. Otangiwai Point is also known as Ngātangiwao, and Letterbox Island (or Letterbox Point) is a local use name.