10. First Report and Recommendations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ICICI Bank Launches 'Social Pay'
VOL-14 ISSUE -6 CONTENTS Editor 2018 Commonwealth Games Ayushman Bharat : National Health Protection Mission N.K. Jain Advisors Neeraj Chabra K.C.Gupta Registered Office Mahendra Publication Pvt. Ltd. 103, Pragatideep Building, Padma Awards 2018 Pulitzer Prize 2018 Plot No. 08, Laxminagar, District Centre, New Delhi - 110092 TIN-09350038898 w.e.f. 12-06-2014 Branch Office Mahendra Publication Pvt. Ltd. E-42,43,44, Sector-7, Noida (U.P.) Interview 5 For queries regarding Current Affairs - One Liner 6-9 promotion, distribution & Spotlight 10 advertisement, contact:- [email protected] The People 11-16 Ph.: 09208037962 News Bites 18-56 Owned, printed & published by World of English - Etymology 57 N.K. Jain Designation : Who's Who 61 103, Pragatideep Building, 2018 Commonwealth Games 63-65 Plot No. 08, Laxminagar, District Centre, New Delhi - 110092 Ayushman Bharat : National Health Protection Mission 66-67 Please send your suggestions and Padma Awards 2018 68-70 grievances to:- Mahendra Publication Pvt. Ltd. Pulitzer Prize 2018 71-72 CP-9, Vijayant Khand, Que Tm - General Awareness 74-84 Gomti Nagar Lucknow - 226010 E-mail:[email protected] RO/ARO 2018 : UPPSC - Solved Paper 2018 85-102 © Copyright Reserved SBI Clerk Pre - Model Paper 2018 103-114 # No part of this issue can be printed in whole or in part without the written permission of the publishers. # All the disputes are subject to Delhi jurisdiction only. Mahendra Publication Pvt. Ltd. Editorial "Courage is what it takes to stand up and speak; courage is also what it takes to sit down and listen." - Winston Churchill Dear Aspirants, Once again we are pleased to present you the 'June 2018' Edition of our very own 'Masters in Current Affairs'. -

Astavarga Plants- Threatened Medicinal Herbs of the North-West Himalaya
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/312533047 Astavarga plants- threatened medicinal herbs of the North-West Himalaya Article · January 2012 CITATIONS READS 39 714 8 authors, including: Anupam Srivastava Rajesh Kumar Mishra Patanjali Research Institute Patanjali Bhartiya Ayurvigyan evum Anusandhan Sansthan 16 PUBLICATIONS 40 CITATIONS 43 PUBLICATIONS 84 CITATIONS SEE PROFILE SEE PROFILE Rajiv K. Vashistha Dr Ajay Singh Hemwati Nandan Bahuguna Garhwal University Patanjali Bhartiya Ayurvigyan Evam Anusandhan Sansthan Haridwar 34 PUBLICATIONS 216 CITATIONS 5 PUBLICATIONS 79 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: ANTI FUNGAL ACTIVITY OF GANDHAK DRUTI AND GANDHAKADYA MALAHAR View project Invivo study of Roscoea purpurea View project All content following this page was uploaded by Rajesh Kumar Mishra on 10 September 2019. The user has requested enhancement of the downloaded file. Int. J. Med. Arom. Plants, ISSN 2249 – 4340 REVIEW ARTICLE Vol. 2, No. 4, pp. 661-676, December 2012 Astavarga plants – threatened medicinal herbs of the North-West Himalaya Acharya BALKRISHNA, Anupam SRIVASTAVA, Rajesh K. MISHRA, Shambhu P. PATEL, Rajiv K. VASHISTHA*, Ajay SINGH, Vikas JADON, Parul SAXENA Patanjali Ayurveda Research and Development Department, Patanjali Yogpeeth, Maharishi Dayanand Gram, Near Bahadrabad, Haridwar- 249405, Uttarakhand, India Article History: Received 24th September 2012, Revised 20th November 2012, Accepted 21st November 2012. Abstract: Astavarga eight medicinal plants viz., Kakoli (Roscoea purpurea Smith), Kshirkakoli (Lilium polyphyllum D. Don), Jeevak (Crepidium acuminatum (D. Don) Szlach), Rishbhak (Malaxis muscifera (Lindl.) Kuntze), Meda (Polygonatum verticillatum (Linn.) Allioni), Mahameda (P. -
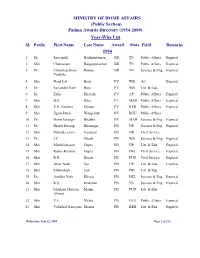
(Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl
MINISTRY OF HOME AFFAIRS (Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl. Prefix First Name Last Name Award State Field Remarks 1954 1 Dr. Sarvapalli Radhakrishnan BR TN Public Affairs Expired 2 Shri Chakravarti Rajagopalachari BR TN Public Affairs Expired 3 Dr. Chandrasekhara Raman BR TN Science & Eng. Expired Venkata 4 Shri Nand Lal Bose PV WB Art Expired 5 Dr. Satyendra Nath Bose PV WB Litt. & Edu. 6 Dr. Zakir Hussain PV AP Public Affairs Expired 7 Shri B.G. Kher PV MAH Public Affairs Expired 8 Shri V.K. Krishna Menon PV KER Public Affairs Expired 9 Shri Jigme Dorji Wangchuk PV BHU Public Affairs 10 Dr. Homi Jehangir Bhabha PB MAH Science & Eng. Expired 11 Dr. Shanti Swarup Bhatnagar PB UP Science & Eng. Expired 12 Shri Mahadeva Iyer Ganapati PB OR Civil Service 13 Dr. J.C. Ghosh PB WB Science & Eng. Expired 14 Shri Maithilisharan Gupta PB UP Litt. & Edu. Expired 15 Shri Radha Krishan Gupta PB DEL Civil Service Expired 16 Shri R.R. Handa PB PUN Civil Service Expired 17 Shri Amar Nath Jha PB UP Litt. & Edu. Expired 18 Shri Malihabadi Josh PB DEL Litt. & Edu. 19 Dr. Ajudhia Nath Khosla PB DEL Science & Eng. Expired 20 Shri K.S. Krishnan PB TN Science & Eng. Expired 21 Shri Moulana Hussain Madni PB PUN Litt. & Edu. Ahmed 22 Shri V.L. Mehta PB GUJ Public Affairs Expired 23 Shri Vallathol Narayana Menon PB KER Litt. & Edu. Expired Wednesday, July 22, 2009 Page 1 of 133 Sl. Prefix First Name Last Name Award State Field Remarks 24 Dr. -

Ayurveda's Immunity Boosting Measures for Self Care During COVID-19 Crisis Among All NSS Functionaries Under Your Jurisdiction
To, All the State NSS Officers, In the region, Punjab, Himachal Pradesh, U.T Chandigarh, Sub:- Ayurveda’s immunity boosting measures for self care during COVID 19 crisis-Reg. Madam/Sir, Kindly find attached herewith the Advisory of Ministry of AYUSH, Government of India received through Directorate of NSS, New Delhi. In this regard It is stated that as you are well aware that in the wake of the Covid 19 outbreak, entire mankind across the globe is suffering. While there is no medicine for COVID-19 as of now, it will be good to take preventive measures which boost our immunity in these times. We all know that prevention is better than cure. So enhancing the body’s natural defence system (immunity) plays an important role in maintaining optimum health. Further It is stated that Ministry of AYUSH recommends the following self-care guidelines for preventive health measures and boosting immunity with special reference to respiratory health. These are supported by Ayurvedic literature and scientific publications. Recommended Measures (I) General Measures 1. Drink warm water throughout the day. 2. Daily practice of Yogasana, Pranayama and meditation for at least 30 minutes as advised by Ministry of AYUSH (#YOGAatHome #StayHome #StaySafe) 3. Spices like Haldi (Turmeric), Jeera (Cumin), Dhaniya (Coriander) and Lahsun (Garlic) are recommended in cooking. (II) Ayurvedic Immunity Promoting Measures 1. Take Chyavanprash 10gm (1tsf) in the morning. Diabetics should take sugar free Chyavanprash. 2. Drink herbal tea / decoction (Kadha) made from Tulsi (Basil), Dalchini (Cinnamon), Kalimirch (Black pepper), Shunthi (Dry Ginger) and Munakka (Raisin) - once or twice a day. -

Global, Regional, and National Age-Sex-Specific Mortality and Life Expectancy, 1950-2017: a Systematic Analysis for the Global Burden of Disease Study 2017
University of Rhode Island DigitalCommons@URI Civil & Environmental Engineering Faculty Publications Civil & Environmental Engineering 11-10-2018 Global, regional, and national age-sex-specific mortality and life expectancy, 1950-2017: A systematic analysis for the Global Burden of Disease Study 2017 Ali S. Akanda University of Rhode Island, [email protected] et al Follow this and additional works at: https://digitalcommons.uri.edu/cve_facpubs Citation/Publisher Attribution Akanda, Ali S., and et al. "Global, regional, and national age-sex-specific mortality and life expectancy, 1950-2017: A systematic analysis for the Global Burden of Disease Study 2017." The Lancet 392, 10159 (2018): 1684-1735. doi:10.1016/S0140-6736(18)31891-9. This Article is brought to you for free and open access by the Civil & Environmental Engineering at DigitalCommons@URI. It has been accepted for inclusion in Civil & Environmental Engineering Faculty Publications by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. Global Health Metrics Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017 GBD 2017 Mortality Collaborators* Summary Lancet 2018; 392: 1684–735 Background Assessments of age-specific mortality and life expectancy have been done by the UN Population Division, This online publication has been Department of Economics and Social Affairs (UNPOP), the United States Census Bureau, WHO, and as part of corrected. The corrected version previous iterations of the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD). Previous iterations of first appeared at thelancet.com the GBD used population estimates from UNPOP, which were not derived in a way that was internally consistent on June 20, 2019 with the estimates of the numbers of deaths in the GBD. -

Magic of the Spices: Elements of Magic Realism in Chitra Banerjee Divakaruni’S the Mistress of Spices
(RJELAL) Research Journal of English Language and Literature Vol.4.Issue 2.2016 A Peer Reviewed (Refereed) International Journal (Apr-Jun) http://www.rjelal.com; Email:[email protected] RESEARCH ARTICLE MAGIC OF THE SPICES: ELEMENTS OF MAGIC REALISM IN CHITRA BANERJEE DIVAKARUNI’S THE MISTRESS OF SPICES Dr. V. SRIVIDHYA1, S. JAYASHREE AGARWAL2 1Assistant Professor of English, National College (Autonomous), Tiruchirappalli 2Assistant Professor of English, Cauvery College for Women, Tiruchirappalli ABSTRACT In this present technologically superfast world, where the click of buttons is changing the way people think, plan and execute their works and live their lives, “virtual reality”, “surrealism”, “hyper reality” are the terms in vogue. They are so commonly used everywhere that they may not seem fanciful any more. However, the excitement in superstitions, magic and mysticism still prevails. India has always caught the imagination of foreigners as the land of hidden secrets – S. JAYASHREE AGARWAL it is viewed as a “hub of cosmic energy” and emerging super power. The secret of Ayurvedas, the variety and depth in art forms like dance and music are equally attractive as Kamasutra and Yoga. The co-existence of various religions, cultures, languages has always been a source of amazement. The festivities, the rituals and the mythological significances keep the attraction alive. Historically significant monuments, the architecture and the cuisine tops the ranking of our country across the globe. In such a context the novel by Chitra Banerjee Divakaruni, The Mistress of Spices (1997), is a delightful read which bombards the senses and tingles the appetite with its exotic use of spices. -
Mental Health Task Force Handout
Let’s join hands to fight against COVID-19 pandemic MENTAL HEALTH TASK FORCE Doctor Harisingh Gour Vishwavidyalaya, Sagar, M.P. Contents in brief Mental and Physical health General Health and care strategies Wellbeing The recent outbreak of COVID-19 pandemic has caused extreme suffering, pain and fear among people across the globe. The World Ayurvedic measures Health Organization (2020) has reported that there is no vaccines or medicines for COVID-19. Thus, prevention is the only measure left Healthy practices that can help us to remain safe from this disease. Based on the recommendations and advisories of the World Health Organization, Mental health protection, Government of India and other specialized bodies, the following are maintenance and promotion recommended to be observed to all the faculty members, non-teaching staff and the students of the University to remain safe and healthy during the pandemic, and even afterwards. Ayurvedic immunity promoting measures for general Health and wellbeing (Ministry of AYUSH, 2020) Take Chyavanprash 10gm (1tsf) in the morning. Diabetics should take sugar-free Chyavanprash. Drink herbal tea/decoction (Kadha) made from Tulsi (Basil), Dalchini (Cinnamon), Kalimirch (Black pepper), Shunthi (Dry Ginger) and Munakka (Raisin) - once or twice a day. Add jaggery (natural sugar) and/or fresh lemon juice to your taste, if needed. Golden Milk- Half teaspoon Haldi (turmeric) powder in 150 ml hot milk - once or twice a day. Spices like Haldi (Turmeric), Jeera (Cumin), Dhaniya (Coriander), Lahsun (Garlic) and Adarak (Ginger) are recommended in cooking. Nasal application - Apply sesame oil/coconut oil or Ghee in both the nostrils (Pratimarsh Nasya) in morning and evening. -

20130118104629Small41.Pdf
Crores of Indian households. A number of family members. Multiple needs. One choice. Joint promoters’ statement “A presence in every house, a recall in every mind – ‘ghar ghar mein Emami’ – has been our driving inspiration since inception” Joint promoters Shri R.S. Agarwal and Shri R.S. Goenka, highlight the Company’s direction and potential There are a number of numerical another year of our successful growth. Emami and growth parameters that one can use to Our performance improved While apparently it appears that 2007- demonstrate that we performed significantly as 2007-08 was a good 08 was tilted towards the industry, the creditably during the financial year year with our topline and bottomline fact remains that we confronted the 2007-08, but the one that stands the growing by 13% and 41% respectively. biggest challenges in terms of an test of industry, analysts and company We feel that there was a sustained unprecedented increase in the cost of is our performance compared with the growth in per capita income, riding on raw materials, a strong price- broad industry average. For instance, the ripple effect of India’s economic sensitivity across most product the broad FMCG industry in India grew growth which benefited a bigger categories, a greater need for wider at a CAGR of about 6% in the last concentration of households. As there and deeper distribution to reach three years; Emami grew at a CAGR was a perceptible increase in people’s customers and the ever-lingering of 25%. aspirations and purchasing power, our threat from counterfeit and This stellar performance sends multiple advertising and brand positioning unorganised products. -

Ministry of Home Affairs Telephone List ********
MINISTRY OF HOME AFFAIRS TELEPHONE LIST ******** HOME MINISTER’S OFFICE NAME & DESIGNATION Room No. OFFICE I.Com RESIDENCE/EMAIL 104 23092462 - - AMIT SHAH 23094686 HOME MINISTER 23017256 (PH) 23017580 (PH)(F) SAKET KUMAR 105 23092462 414 - PS TO HM 23094686 23094221(FAX) RAJEEV KUMAR 107 23092462 414 - OSD in HM Office 23094686 23092113(FAX) SATISH KUMAR CHHIKARA 23092462 414 - OSD in HM Office 23094686 23092979(FAX) ABHISHEK M. CHAUDHARI 103-A 23092462 414 - APS TO HM VIJAY KUMAR UPADHYAY 10-C 23093038 487 - DS (HMO) Dr. RAM MOHAN SHARMA R. No. 01 23075183 787 - Legal Advisor (HM Office) MDCNS 23072674(FAX) MINISTER OF STATE (N) OFFICE NITYANAND RAI 101 23092870 - - MINISTER OF STATE 23092595 SURESH KUMAR 198 23092595 270 - DIRECTOR 23094896(FAX) PS TO MoS(N) KUMAR KRANTI 196-A 23092870 270 - ADDL.PS TO MOS 23094896(FAX) ALOK KUMAR 117-B 23092595 270 - PPS O/o MoS MINISTER OF STATE(AM) OFFICE AJAY KUMAR MISHRA 127 23092073 - MINISTER OF STATE 23094054 ANANT KISHORE SARAN 127-B 23092073 318 [email protected] DIR O/o MoS (AM) 23094054 YOGESH SHARMA 125-B 23092073 318 [email protected] PSO O/o MoS (AM) 23094054 SHIV KUMAR 125-B 23092073 ̘318 US O/o MoS(AM) 23094495 23093549(FAX) AMIT JAIN 126-C 23092073 318 PS O/o MoS(AM) 23094054 23094495 23093549(FAX) RAJESH P. DALAL 126-C 23092073 318 SO O/o MoS(AM) 23094054 23093549(FAX) MINISTER OF STATE(NP) OFFICE NISITH PRAMANIK 127-A 23092123 - MINISTER OF STATE 23093143 MUKESH KUMAR 126-A 23092123 432 PPS 23093143 SANDEEP AMBASTHA 126-A 23092123 432 PS 23093143 HOME SECRETARY’S OFFICE AJAY KUMAR BHALLA 113 23092989 215 [email protected] HOME SECRETARY 23093031 23093003 (FAX) M JAGANNATHA RAO 113-A 23092989 254 [email protected] Sr. -

Chyavanprash: India's Most Popular Herbal Tonic
ChyavanprashIndia’s most popular herbal tonic Chyavanprash, sometimes called “the elixir of life”, is the most popular Rasayana (health tonic) in India. In the ancient epic called the Mahabharata, is Out of curiosity she stuck the two shining things with a a fascinating story of the rejuvenation of the thornChya and mistakenly blinded the ascetic. vanprash - the ‘elixir of life’ elderly Vedic seer, Chyavana and of the origins of Chyavanprash. A Rishi’s curse The sudden pain this produced, brought Chyavana Rishi The ant-hill Rishi abruptly out of his meditation and he became filled with From birth Chyavana was said to be physically anger. The saint cursed the army of Saryati and all the weak, but he had a strong spiritual drive that king’s men became filled with physical distress. lead him to devote many years of his life to For a long time the king sought the cause of this prolonged meditation practices. One such misfortune, and when he finally discovered the offence practice lasted so long that a mound of earth his daughter had committed, he immediately went to formed over him, on which ants crawled about. To all Chyavana to obtain his forgiveness. appearances, Chyavana appeared like an anthill. Chyavana said that he would only become reconciled if the king King Saryati’s beautiful young daughter Sukanya, gave him his daughter as a wife. So the young Saryati married romping about the forest with her playmates discovered this frail old man. the anthill, in which only the two eyes of the ascetic were visible. To the girl, the eyes appeared like glow-worms. -

Development of Quality Control Methods for Polyherbal Formulation, Chyawanprash
Researth Artitle Development of quality control methods for polyherbal formulation, Chyawanprash RaIlul P Kasar1, K S Laddha1*,Jayesh Chaudhary2 and Anil Shulda2 IMedicinal Natural Products Research Laboratory, Pharmaceutical Division University Institute of Chemical Technology, N M Parikh Marg, Matunga (E), Mumbai- 400 019, Maharashtra, India 2VedicLife Sciences, 118 Morya House, OffNew Link Road, Andheri (W), Mumbai-400 053 *Correspondent author, E-mail: [email protected]; [email protected] Received 22 March 2005; Accepted 12 September 2005 Abstract the harmful effectof stress2• In the modern Chyawanprash is a traditional polyherbal formulation, which is widely used as tonic, time it cures cough, asthma and act as an rejuvenator, anabolic, immunomodulator and memory enhancer. Chyawanprash contains the pulp immunomodulator and memory of Emblka officinalis Gaertn. as the prime ingredient, along with powder and extract of enhancer3,4. It promotes growth in several other herbs. It is observed that the consistency and taste varies from one manufacturer to chUdren, increases the sexual power and another. Even these variations are observed in the same pharmaceutical company in different manufacturing batches. Hence, it is the need of the hour to standardize the raw materials to obtain digestive fire. The entire Indian product consistency. With the advent of new analytical tools and sophisticated instrmpental Chyawanprash market stands around 50• technology, the quality assurance profile for a crude drug or its bioactive constituents can be made 60 tonnes5, even though only very few possible. 'I\voin-house batch ofChyawanprash was prepared according to the procedure described quality control methods for its evaluation in text Charaksamhita and one Chyawanprash sample was procured from market. -

Consolidated List of Article 13 Health Claims List of References Received by EFSA
Unit on Dietetic Products, Nutrition and Allergies Parma, 5 April 2011 Consolidated list of Article 13 health claims List of references received by EFSA Part 3 IDs 2001 – 3000 (This document contains the list of references for claims which the Commission has asked EFSA to prioritise in the evaluation.) BACKGROUND In accordance with Article 13 of Regulation (EC) No 1924/20061 Member States had provided the European Commission with lists of claims accompanied by the conditions applying to them and by references to the relevant scientific justification by 31 January 2008. EFSA has received from the European Commission nine Access databases with a consolidated list of 4,185 main health claim entries with around 10,000 similar health claims. The similar health claims were accompanied by the conditions of use and scientific references. The nine Access databases were sent in three batches - in July 2008, in November 2008 and in December 2008. Subsequently, EFSA combined the databases into one master database and re-allocated upon request of the Commission and Member States similar health claims which had been accidentally placed under a wrong main health claim entry (misplaced claims). During this process some Member States also identified a number of similar health claims which still needed to be submitted to EFSA (―missing claims‖). These similar claims were also added to the database. In March 2010, the European Commission forwarded to EFSA an addendum to the consolidated list containing an additional 452 main entry claims which have been added to the updated final database which was published on the EFSA website in May 2010 (containing 4,637 main entry claims).