Climate Change and Water-Related Infectious Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Balantidium Coli
GLOBAL WATER PATHOGEN PROJECT PART THREE. SPECIFIC EXCRETED PATHOGENS: ENVIRONMENTAL AND EPIDEMIOLOGY ASPECTS BALANTIDIUM COLI Francisco Ponce-Gordo Complutense University Madrid, Spain Kateřina Jirků-Pomajbíková Institute of Parasitology Biology Centre, ASCR, v.v.i. Budweis, Czech Republic Copyright: This publication is available in Open Access under the Attribution-ShareAlike 3.0 IGO (CC-BY-SA 3.0 IGO) license (http://creativecommons.org/licenses/by-sa/3.0/igo). By using the content of this publication, the users accept to be bound by the terms of use of the UNESCO Open Access Repository (http://www.unesco.org/openaccess/terms-use-ccbysa-en). Disclaimer: The designations employed and the presentation of material throughout this publication do not imply the expression of any opinion whatsoever on the part of UNESCO concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The ideas and opinions expressed in this publication are those of the authors; they are not necessarily those of UNESCO and do not commit the Organization. Citation: Ponce-Gordo, F., Jirků-Pomajbíková, K. 2017. Balantidium coli. In: J.B. Rose and B. Jiménez-Cisneros, (eds) Global Water Pathogens Project. http://www.waterpathogens.org (R. Fayer and W. Jakubowski, (eds) Part 3 Protists) http://www.waterpathogens.org/book/balantidium-coli Michigan State University, E. Lansing, MI, UNESCO. Acknowledgements: K.R.L. Young, Project Design editor; Website Design (http://www.agroknow.com) Published: January 15, 2015, 11:50 am, Updated: October 18, 2017, 5:43 pm Balantidium coli Summary 1.1.1 Global distribution Balantidium coli is reported worldwide although it is To date, Balantidium coli is the only ciliate protozoan more common in temperate and tropical regions (Areán and reported to infect the gastrointestinal track of humans. -

New Zealand's Genetic Diversity
1.13 NEW ZEALAND’S GENETIC DIVERSITY NEW ZEALAND’S GENETIC DIVERSITY Dennis P. Gordon National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington 6022, New Zealand ABSTRACT: The known genetic diversity represented by the New Zealand biota is reviewed and summarised, largely based on a recently published New Zealand inventory of biodiversity. All kingdoms and eukaryote phyla are covered, updated to refl ect the latest phylogenetic view of Eukaryota. The total known biota comprises a nominal 57 406 species (c. 48 640 described). Subtraction of the 4889 naturalised-alien species gives a biota of 52 517 native species. A minimum (the status of a number of the unnamed species is uncertain) of 27 380 (52%) of these species are endemic (cf. 26% for Fungi, 38% for all marine species, 46% for marine Animalia, 68% for all Animalia, 78% for vascular plants and 91% for terrestrial Animalia). In passing, examples are given both of the roles of the major taxa in providing ecosystem services and of the use of genetic resources in the New Zealand economy. Key words: Animalia, Chromista, freshwater, Fungi, genetic diversity, marine, New Zealand, Prokaryota, Protozoa, terrestrial. INTRODUCTION Article 10b of the CBD calls for signatories to ‘Adopt The original brief for this chapter was to review New Zealand’s measures relating to the use of biological resources [i.e. genetic genetic resources. The OECD defi nition of genetic resources resources] to avoid or minimize adverse impacts on biological is ‘genetic material of plants, animals or micro-organisms of diversity [e.g. genetic diversity]’ (my parentheses). -

Wda 2016 Conference Proceedi
The 65th International Conference of the Wildlife Disease Association July 31 August 5, 2016 Cortland, New York Sustainable Wildlife: Health Matters! Hosted by at Greek Peak Mountain Resort Cortland, New York, USA 4 Wildlife Disease Association Table of Contents Welcome from the Conference Committee 6 Conference Committee Members 7 WDA Officers 8 Plenary Speakers 9 The 2016 Carlton Herman Fund Speaker 11 Sponsors 12 Events & Meetings 15 Pre-conference Workshops 17 Program 21 Poster Sessions 34 Abstracts 40 Exhibitors 249 Index 256 65th Annual International Conference 5 Welcome from the Conference Committee Welcome to beautiful central New York, the 65th International Conference of the Wildlife Disease Association, and the 2016 Annual Meeting of the American Association of Wildlife Veterinarians. We have planned an exciting scientific program and have lots of networking and learning opportunities in store this week. There will be a break mid-week to allow time to socialize with new and old friends and take in some of the adventures the area has to offer. global challenges and opportunities in managing wildlife health issues with our excellent slate of plenary speakers. In addition, we have three special sessions: How Risk Communication Research in Wildlife Disease Management Contributes to Wildlife Trust Administration, Chelonian Diseases and Conservation, and Vaccines for Conservation. This meeting would not have been possible without the contributions of numerous people and institutions. We are grateful to Cornell University for in-kind and financial support for this event. The Atkinson Center for a Sustainable Future, College of Veterinary Medicine, Department of Population Medicine and Diagnostic Sciences, Animal Health Diagnostic Center, and Lab of Ornithology have all been integral parts of making this meeting a success. -

Co-Infection Associated with Diarrhea in a Colony of <I>Scid
Laboratory Animal Science Vol 48, No 5 Copyright 1998 October 1998 by the American Association for Laboratory Animal Science Helicobacter bilis/Helicobacter rodentium Co-Infection Associated with Diarrhea in a Colony of scid Mice Nirah H. Shomer,* Charles A. Dangler, Robert P. Marini, and James G. Fox† Abstract _ An outbreak of diarrhea spanning 3 months occurred in a breeding colony of scid/Trp53 knockout mice. Approximately a third of the 150 mice were clinically affected, with signs ranging from mucoid or watery diarrhea to severe hemorrhagic diarrhea with mortality. Helicobacter bilis and the newly recognized urease-negative organ- ism H. rodentium were isolated from microaerobic culture of feces or cecal specimens from affected mice. Dual infection with H. bilis and H. rodentium were confirmed by culture and polymerase chain reaction (PCR) in several animals. Both Helicobacter species rapidly colonized immunocompetent sentinel mice exposed to bedding from cages containing affected mice, but the sentinel remained asymptomatic. Mice with diarrhea had multifocal to segmental proliferative typhlitis, colitis, and proctitis. Several affected mice had multifocal mucosal necrosis with a few focal ulcers in the cecum, colon, and rectum. Mice with diarrhea were treated with antibiotic food wafers (1.5 mg of amoxicillin, 0.69 mg of metronidazole, and 0.185 mg of bismuth/mouse per day) previously shown to eradi- cate H. hepaticus in immunocompetent mice. Antibiotic treatment resulted in resolution of diarrhea, but not eradication of H. bilis and H. rodentium; mice continued to have positive PCR results after a 2-week treatment regimen, and clinical signs of diarrhea returned in some mice when treatment was suspended. -

Enteric Protozoa in the Developed World: a Public Health Perspective
Enteric Protozoa in the Developed World: a Public Health Perspective Stephanie M. Fletcher,a Damien Stark,b,c John Harkness,b,c and John Ellisa,b The ithree Institute, University of Technology Sydney, Sydney, NSW, Australiaa; School of Medical and Molecular Biosciences, University of Technology Sydney, Sydney, NSW, Australiab; and St. Vincent’s Hospital, Sydney, Division of Microbiology, SydPath, Darlinghurst, NSW, Australiac INTRODUCTION ............................................................................................................................................420 Distribution in Developed Countries .....................................................................................................................421 EPIDEMIOLOGY, DIAGNOSIS, AND TREATMENT ..........................................................................................................421 Cryptosporidium Species..................................................................................................................................421 Dientamoeba fragilis ......................................................................................................................................427 Entamoeba Species.......................................................................................................................................427 Giardia intestinalis.........................................................................................................................................429 Cyclospora cayetanensis...................................................................................................................................430 -

Enterohepatic Lesions in SCID Mice Infected with Helicobacter Bilis
Laboratory Animal Science Vol 48, No 4 Copyright 1998 August 1998 by the American Association for Laboratory Animal Science Enterohepatic Lesions in SCID Mice Infected with Helicobacter bilis Craig L. Franklin, Lela K. Riley, Robert S. Livingston, Catherine S. Beckwith, Cynthia L. Besch-Williford, and Reuel R. Hook, Jr. Abstract _ Helicobacter bilis is a recently identified species that colonizes the intestine and liver of mice. In immunocompetent mice, infections have been associated with mild hepatitis, and in immunocompromised mice, inflammatory bowel disease has been induced by intraperitoneal inoculation of the organism. We re- port inoculation of 6-week-old C.B-17 scid/scid mice by gastric gavage with approximately 107 H. bilis colony- forming units. Groups of mice were euthanized and necropsied 12, 24, and 36 weeks after inoculation. Mild to moderate proliferative typhlitis was evident in all mice at 12 and 36 weeks after inoculation and in most mice 24 weeks after inoculation. Mild to severe chronic active hepatitis was detected in 10 of 10 male mice and 3 of 10 female mice. These results indicate that H. bilis can cause moderate to severe enterohepatic disease in immunocompromised mice. The genus Helicobacter is a rapidly expanding genus volved in lesion development. Culture of specimens from currently containing 17 named species. Members of this mice confirmed intestinal colonization with H. hepaticus. Fox genus are microaerophilic, have curved to spiral rod mor- et al. reported enteric lesions in immunocompetent germ- phology, and are motile by flagella that vary in number free Swiss Webster mice infected with H. hepaticus (15), and and location among various species (1). -

Biliary Tract Microbiota: a New Kid on the Block of Liver Diseases?
European Review for Medical and Pharmacological Sciences 2020; 24: 2750-2775 Biliary tract microbiota: a new kid on the block of liver diseases? A. NICOLETTI1, F.R. PONZIANI2, E. NARDELLA1, G. IANIRO2, A. GASBARRINI1, L. ZILERI DAL VERME2 1Internal Medicine, Gastroenterology and Hepatology, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy 2Internal Medicine, Gastroenterology and Hepatology, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy Abstract. – The microbiome plays a crucial man body1,2. Indeed, a resident microbiota has recent- role in maintaining the homeostasis of the or- ly been described in several human environments ganism. Recent evidence has provided novel previously described as devoid of microorganisms, insights for understanding the interaction be- such as the urinary tract and the stomach3-9. Even tween the microbiota and the host. However, the 10 vast majority of such studies have analyzed the healthy placenta hosts microbial communities . interactions taking place in the intestinal tract. Bile has traditionally been considered sterile The biliary tree has traditionally been consid- under normal conditions11-14. ered sterile under normal conditions. However, The physical and chemical features of bile and the advent of metagenomic techniques has re- its antimicrobial activity were supposed to create vealed an unexpectedly rich bacterial communi- a hostile environment for bacteria. Moreover, the ty in the biliary tract. Associations between specific microbiolog- difficulty in collecting bile samples, coupled with ical patterns and inflammatory biliary diseases the lack of sensibility of culture techniques in and cancer have been recently described. Hence, detecting microbes in low-charge samples, sus- biliary dysbiosis may be a primary trigger in the tained this hypothesis for a long time. -

Catalogue of Protozoan Parasites Recorded in Australia Peter J. O
1 CATALOGUE OF PROTOZOAN PARASITES RECORDED IN AUSTRALIA PETER J. O’DONOGHUE & ROBERT D. ADLARD O’Donoghue, P.J. & Adlard, R.D. 2000 02 29: Catalogue of protozoan parasites recorded in Australia. Memoirs of the Queensland Museum 45(1):1-164. Brisbane. ISSN 0079-8835. Published reports of protozoan species from Australian animals have been compiled into a host- parasite checklist, a parasite-host checklist and a cross-referenced bibliography. Protozoa listed include parasites, commensals and symbionts but free-living species have been excluded. Over 590 protozoan species are listed including amoebae, flagellates, ciliates and ‘sporozoa’ (the latter comprising apicomplexans, microsporans, myxozoans, haplosporidians and paramyxeans). Organisms are recorded in association with some 520 hosts including mammals, marsupials, birds, reptiles, amphibians, fish and invertebrates. Information has been abstracted from over 1,270 scientific publications predating 1999 and all records include taxonomic authorities, synonyms, common names, sites of infection within hosts and geographic locations. Protozoa, parasite checklist, host checklist, bibliography, Australia. Peter J. O’Donoghue, Department of Microbiology and Parasitology, The University of Queensland, St Lucia 4072, Australia; Robert D. Adlard, Protozoa Section, Queensland Museum, PO Box 3300, South Brisbane 4101, Australia; 31 January 2000. CONTENTS the literature for reports relevant to contemporary studies. Such problems could be avoided if all previous HOST-PARASITE CHECKLIST 5 records were consolidated into a single database. Most Mammals 5 researchers currently avail themselves of various Reptiles 21 electronic database and abstracting services but none Amphibians 26 include literature published earlier than 1985 and not all Birds 34 journal titles are covered in their databases. Fish 44 Invertebrates 54 Several catalogues of parasites in Australian PARASITE-HOST CHECKLIST 63 hosts have previously been published. -

U.S. Environmental Protection Agency Relative Risk Assessment Of
United States Environmental Protection Agency Relative Risk Assessment of Management Options for Treated Wastewater in South Florida Office of Water (4606M) EPA 816-R-03-010 www.epa.gov/safewater Recycled/Recyclable • Printed with Vegetable Oil Based Inks on April 2003 Recycled Paper (Minimum 30% Postconsumer) [ This page intentionally left blank ] TABLE OF CONTENTS Page EXECUTIVE SUMMARY ES-1 Wastewater Challenges in South Florida ES-1 Congressional Mandate for Relative Risk Assessment ES-2 Municipal Wastewater Treatment Options in South Florida ES-2 Wastewater Treatment Options ES-3 Levels of Wastewater Treatment and Disinfection ES-4 Risk Assessment ES-5 Approach Used in this Relative Risk Assessment ES-6 Deep-Well Injection ES-7 Regulatory Oversight of Deep-Well Injection ES-10 Option-Specific Risk Analysis for Deep-Well Injection ES-11 How Injected Wastewater Can Reach Drinking-Water Supplies ES-11 Human Health and Ecological Risk Characterization of Deep-Well Injection ES-13 Aquifer Recharge ES-14 Regulatory Oversight of Aquifer Recharge ES-14 Option-Specific Risk Analysis for Aquifer Recharge ES-15 Human Health and Ecological Risk Characterization of Aquifer Recharge ES-16 Discharge to Ocean Outfalls ES-16 Regulatory Oversight of Discharge to Ocean Outfalls ES-17 Option-Specific Risk Analysis for Discharge to Ocean Outfalls ES-18 Human Health and Ecological Risk Characterization of Discharge to Ocean Outfalls ES-19 Discharge to Surface Waters ES-19 Regulatory Oversight of Discharge to Surface Waters ES-20 Option-Specific Risk Analysis -
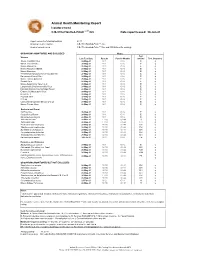
2021 ARC Health Report
Animal Health Monitoring Report Isolator reared C.B-17/IcrHanHsd-Prkdc scid /Arc Date report issued: 30-Jun-21 Report covers the following isolators: RI 27 Strains present in isolator: C.B-17/IcrHanHsd-Prkdc scid /Arc Strain of animal tested: C.B-17/IcrHanHsd-Prkdc scid /Arc and ICR Outbred for serology ORGANISMS MONITORED AND EXCLUDED Mouse Test Viruses Last Test Date Results Past 18 Months method Test frequency Mouse Hepatitis Virus 26-May-21 0 / 1 0 / 6 E q Minute Virus of Mice 26-May-21 0 / 1 0 / 6 E q Mouse Parvovirus 26-May-21 0 / 1 0 / 6 E q Murine Rotavirus (EDIM) 26-May-21 0 / 1 0 / 6 E q Mouse Norovirus 26-May-21 0 / 1 0 / 6 E q Theiler's Encephalomyelitis Virus (GD VII) 26-May-21 0 / 1 0 / 6 E q Pneumonia Virus of Mice 26-May-21 0 / 1 0 / 6 E q Murine Cytomegalovirus 26-May-21 0 / 1 0 / 6 E q Sendai Virus 26-May-21 0 / 1 0 / 6 E q Mouse Adenovirus Type 1 & 2 26-May-21 0 / 1 0 / 6 E q Lymphocytic Choriomeningitis Virus 26-May-21 0 / 1 0 / 6 E q Hantaan (Korean Haemorrhagic Fever) 26-May-21 0 / 1 0 / 6 E q Ectromelia (Mousepox) Virus 26-May-21 0 / 1 0 / 6 E q Reovirus -3 26-May-21 0 / 1 0 / 6 E q Polyoma Virus 26-May-21 0 / 1 0 / 6 E q K Virus 26-May-21 0 / 1 0 / 6 E q Lactic Dehydrogenase Elevating Virus 26-May-21 0 / 1 0 / 6 E q Mouse Thymic Virus 26-May-21 0 / 1 0 / 6 E q 00-Jan-00 Bacteria and Fungi CAR bacillus 26-May-21 0 / 1 0 / 6 E q Clostridium piliforme 26-May-21 0 / 1 0 / 6 E q Mycoplasma pulmonis 26-May-21 0 / 1 0 / 6 E q Helicobacter spp.1 26-May-21 0 / 10 0 / 44 H q Salmonella spp. -
R Graphics Output
883 | Desulfovibrio vulgaris | DvMF_2825 298701 | Desulfovibrio | DA2_3337 1121434 | Halodesulfovibrio aestuarii | AULY01000007_gene1045 207559 | Desulfovibrio alaskensis | Dde_0991 935942 | Desulfonatronum lacustre | KI912608_gene2193 159290 | Desulfonatronum | JPIK01000018_gene1259 1121448 | Desulfovibrio gigas | DGI_0655 1121445 | Desulfovibrio desulfuricans | ATUZ01000018_gene2316 525146 | Desulfovibrio desulfuricans | Ddes_0159 665942 | Desulfovibrio | HMPREF1022_02168 457398 | Desulfovibrio | HMPREF0326_00453 363253 | Lawsonia intracellularis | LI0397 882 | Desulfovibrio vulgaris | DVU_0784 1121413 | Desulfonatronovibrio hydrogenovorans | JMKT01000008_gene1463 555779 | Desulfonatronospira thiodismutans | Dthio_PD0935 690850 | Desulfovibrio africanus | Desaf_1578 643562 | Pseudodesulfovibrio aespoeensis | Daes_3115 1322246 | Pseudodesulfovibrio piezophilus | BN4_12523 641491 | Desulfovibrio desulfuricans | DND132_2573 1121440 | Desulfovibrio aminophilus | AUMA01000002_gene2198 1121456 | Desulfovibrio longus | ATVA01000018_gene290 526222 | Desulfovibrio salexigens | Desal_3460 1121451 | Desulfovibrio hydrothermalis | DESAM_21057 1121447 | Desulfovibrio frigidus | JONL01000008_gene3531 1121441 | Desulfovibrio bastinii | AUCX01000006_gene918 1121439 | Desulfovibrio alkalitolerans | dsat_0220 941449 | Desulfovibrio | dsx2_0067 1307759 | Desulfovibrio | JOMJ01000003_gene2163 1121406 | Desulfocurvus vexinensis | JAEX01000012_gene687 1304872 | Desulfovibrio magneticus | JAGC01000003_gene2904 573370 | Desulfovibrio magneticus | DMR_04750 -

Balantidium Coli
Intestinal Ciliates, Coccidia (Sporozoa) and Microsporidia Balantidium coli (Ciliates) Cyclospora cayetanensis (Coccidia, Sporozoa) Cryptosporidium parvum (Coccidia, Sporozoa) Enterocytozoon bieneusi (Microsporidia) Isospora belli (Coccidia, Sporozoa) Encephalitozoon intestinalis (Microsporidia) Balantidium coli Introduction: Balantidium coli is the only member of the ciliate (Ciliophora) family to cause human disease (Balantadiasis). It is the largest known protozoan causing human infection. Morphology: Trophozoites: Trophozoites are oval in shape and measure approximately 30-200 um. The main morphological characteristics are cilia, 2 types of nuclei; 1 macro (Kidney shaped) and 1 micronucleus and a large contractile vacuole. A funnel shaped cytosome can be seen near the anterior end. Cysts: The cyst is spherical or round ellipsoid and measures from 30-70 um. It contains 1 macro and 1 micronucleus. The cilia are present in young cysts and may be seen slowly rotating, but after prolonged encystment, the cilia disappear. Life Cycle: Definitive hosts are humans including a main reservoir are the pigs (It is estimated that 63-90 % harbor B. coli), rodents and other animals with no intermidiate hosts or vectors. Cysts (Infective stage) are responsible for transmission of balantidiasis through ingestion of contaminated food or water through the oral-fecal route. Following ingestion, excystation occurs in the small intestine, and the trophozoites colonize the large intestine. The trophozoites reside in the lumen of the large intestine of humans and animals, where they replicate by binary fission. Some trophozoites invade the wall of the colon and Parasitology Chapter 4 The Ciliates and Coccidia Page 1 of 9 multiply. Trophozoites undergo encystation to produce infective cysts. Mature cysts are passed with feces.