Threatened Habitat Types in Finland 2018 Red List of Habitats Results and Basis for Assessment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uimarantaluettelo 2021
Uimarantaluettelo 1 (3) 7.5.2021 UUDENKAPUNGIN YMPÄRISTÖTERVEYDENHUOLLON UIMARANTALUETTELO VUODELLE 2021 (Kustavi, Laitila, Masku, Mynämäki, Nousiainen, Pyhäranta, Tai- vassalo, Uusikaupunki ja Vehmaa) Kunnan terveydensuojeluviranomaisen on Sosiaali- ja terveysministeriön asetusten 177/2008 4 §:n (yleinen uimaranta) ja asetuksen 354/2008 4§:n (pieni yleinen uimaran- ta) mukaan laadittava uimarantaluettelo asetusten soveltamisalaan kuuluvista uimaran- noista. Yleisellä uimarannalla eli EU-uimarannalla tarkoitetaan uimarantaa, jossa kunnan ter- veydensuojeluviranomaisen määrittelyn mukaan odotetaan huomattavan määrän ih- misiä uivan uimakauden (15.6.-31.8.) aikana. Pienellä yleisellä uimarannalla taas tar- koitetaan yleistä uimarantaa, jossa vastaavan arvion mukaan ei odoteta huomattavan määrän ihmisiä uivan uimakauden aikana. Yleisten uimarantojen veden laatua ja sinilevien esiintymistä valvotaan uimakaudella säännöllisesti. EU-uimarannoilta ensimmäinen uimavesinäyte otetaan noin kaksi viikkoa ennen uimakauden alkua. Tämän lisäksi uimakauden aikana otetaan ja analysoidaan vähintään kolme näytettä. Pieniltä yleisiltä uimarannoilta otetaan ja analysoidaan uima- kauden aikana vähintään kolme näytettä. Kunnan terveydensuojeluviranomaisen on huolehdittava siitä, että kunnan asukkailla ja kesäasukkailla on mahdollisuus saada tietoa sekä tehdä ehdotuksia ja huomautuksia sekä yleisten uimarantojen että pienten yleisten uimarantojen uimarantaluettelosta. Yleisöllä on mahdollisuus tehdä ehdotuksia ja huomautuksia uimarantaluettelosta. Ne tulee tehdä -
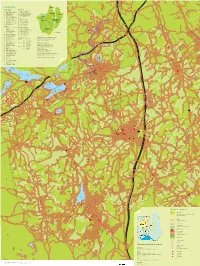
Nurmijärven Matkailu Ja Ulkoilukartta
NURMIJÄRVI NÄHTÄVYYDET RATSASTUS 1 Aleksis Kiven patsas 39 Islanninhevostalli Dynur 2 Aleksis Kiven syntymäkoti 55 Mintzun issikkatalli 3 Aleksis Kivi -huone 42 Nurmijärven Ratsastuskoulu 4 Klaukkalan kirkko 41 Perttulan Ratsastuskeskus 5 Museokahvila 44 Ratsastuskoulu Solbacka 6 Nukarin koulumuseo 53 Vuonotalli Solsikke 3 Nurmijärven Kirjastogalleria 7 Nurmijärven kirkko UINTI 2 Puupiirtäjän työhuone 45 Herusten uimapaikka 8 Pyhän Nektarioksen kirkko 46 Röykän uimapaikka 9 Rajamäen kirkko 35 Sääksjärven uimaranta 10 Taaborinvuoren museoalue 47 Tiiran uimaranta 11 Vanha hautausmaa 48 Vaaksin uimapaikka 49 Rajamäen uimahalli PALVELUT 12 Nurmijärven kunnanvirasto MAJOITUSPAIKKOJA Kirjastot 32 Holman kurssikeskus 13 Klaukkalan kirjasto 50 Hyvinvointikeskus Haukilampi 3 Nurmijärven pääkirjasto 34 Ketolan strutsitila 14 Rajamäen kirjasto 15 Hotelli Kiljavanranta 51 Lomakoti Kotoranta ELÄMYKSIÄ JA LUONTOA 54 Nukarin lomamökit 18 Alhonniitun ulkoilualue 53 Vuonotalli Solsikke 52 Ali-Ollin Alpakkatila 56 Bowling House TYÖPAIKKA-ALUEET MITEN MERKATAAN KUNTORADAT? 25 Maaniitun-Ihantolan Klaukkala A Järvihaka Kuntoratakartan värikoodit ei toimi tässä. liikuntapuisto B Pietarinmäki Numeroinnilla pelkästään?... 30 Frisbeegolfrata C Ristipakka Rajamäen liikuntapuisto Nurmijärvi D Alhonniittu 19 Isokallion ulkoilualue E Ilvesvuori Rajamäki – Kehityksen maja 20 Klaukkalan jäähalli F Karhunkorpi Kehityksen Majan kilpalatu 21 Klaukkalan urheilualue G Kuusimäki Märkiön lenkki (Kehityksen majalta) 22 Tarinakoru Rajamäki H Ketunpesä Matkun lenkki (Kehityksen -

Torches and Torch Relays of the Olympic Summer Games from Berlin 1936 to London 2012 Reference Document
Olympic Studies Centre Torches and Torch Relays of the Olympic Summer Games from Berlin 1936 to London 2012 Reference document Presentation and visuals of the Olympic torches. Facts and figures on the Torch Relay for each edition. November 2014 © IOC – John HUET Reference document TABLE OF CONTENTS Introduction .................................................................................................................. 3 Berlin 1936 ................................................................................................................... 5 London 1948 ................................................................................................................ 9 Helsinki 1952 .............................................................................................................. 13 Melbourne/Stockholm 1956 ...................................................................................... 17 Rome 1960 .................................................................................................................. 23 Tokyo 1964 ................................................................................................................. 27 Mexico City 1968 ........................................................................................................ 31 Munich 1972 ............................................................................................................... 35 Montreal 1976 ............................................................................................................ -

Recent Noteworthy Findings of Fungus Gnats from Finland and Northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae)
Biodiversity Data Journal 2: e1068 doi: 10.3897/BDJ.2.e1068 Taxonomic paper Recent noteworthy findings of fungus gnats from Finland and northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae) Jevgeni Jakovlev†, Jukka Salmela ‡,§, Alexei Polevoi|, Jouni Penttinen ¶, Noora-Annukka Vartija# † Finnish Environment Insitutute, Helsinki, Finland ‡ Metsähallitus (Natural Heritage Services), Rovaniemi, Finland § Zoological Museum, University of Turku, Turku, Finland | Forest Research Institute KarRC RAS, Petrozavodsk, Russia ¶ Metsähallitus (Natural Heritage Services), Jyväskylä, Finland # Toivakka, Myllyntie, Finland Corresponding author: Jukka Salmela ([email protected]) Academic editor: Vladimir Blagoderov Received: 10 Feb 2014 | Accepted: 01 Apr 2014 | Published: 02 Apr 2014 Citation: Jakovlev J, Salmela J, Polevoi A, Penttinen J, Vartija N (2014) Recent noteworthy findings of fungus gnats from Finland and northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae). Biodiversity Data Journal 2: e1068. doi: 10.3897/BDJ.2.e1068 Abstract New faunistic data on fungus gnats (Diptera: Sciaroidea excluding Sciaridae) from Finland and NW Russia (Karelia and Murmansk Region) are presented. A total of 64 and 34 species are reported for the first time form Finland and Russian Karelia, respectively. Nine of the species are also new for the European fauna: Mycomya shewelli Väisänen, 1984,M. thula Väisänen, 1984, Acnemia trifida Zaitzev, 1982, Coelosia gracilis Johannsen, 1912, Orfelia krivosheinae Zaitzev, 1994, Mycetophila biformis Maximova, 2002, M. monstera Maximova, 2002, M. uschaica Subbotina & Maximova, 2011 and Trichonta palustris Maximova, 2002. Keywords Sciaroidea, Fennoscandia, faunistics © Jakovlev J et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

Helsinki in Early Twentieth-Century Literature Urban Experiences in Finnish Prose Fiction 1890–1940
lieven ameel Helsinki in Early Twentieth-Century Literature Urban Experiences in Finnish Prose Fiction 1890–1940 Studia Fennica Litteraria The Finnish Literature Society (SKS) was founded in 1831 and has, from the very beginning, engaged in publishing operations. It nowadays publishes literature in the fields of ethnology and folkloristics, linguistics, literary research and cultural history. The first volume of the Studia Fennica series appeared in 1933. Since 1992, the series has been divided into three thematic subseries: Ethnologica, Folkloristica and Linguistica. Two additional subseries were formed in 2002, Historica and Litteraria. The subseries Anthropologica was formed in 2007. In addition to its publishing activities, the Finnish Literature Society maintains research activities and infrastructures, an archive containing folklore and literary collections, a research library and promotes Finnish literature abroad. Studia fennica editorial board Pasi Ihalainen, Professor, University of Jyväskylä, Finland Timo Kaartinen, Title of Docent, Lecturer, University of Helsinki, Finland Taru Nordlund, Title of Docent, Lecturer, University of Helsinki, Finland Riikka Rossi, Title of Docent, Researcher, University of Helsinki, Finland Katriina Siivonen, Substitute Professor, University of Helsinki, Finland Lotte Tarkka, Professor, University of Helsinki, Finland Tuomas M. S. Lehtonen, Secretary General, Dr. Phil., Finnish Literature Society, Finland Tero Norkola, Publishing Director, Finnish Literature Society Maija Hakala, Secretary of the Board, Finnish Literature Society, Finland Editorial Office SKS P.O. Box 259 FI-00171 Helsinki www.finlit.fi Lieven Ameel Helsinki in Early Twentieth- Century Literature Urban Experiences in Finnish Prose Fiction 1890–1940 Finnish Literature Society · SKS · Helsinki Studia Fennica Litteraria 8 The publication has undergone a peer review. The open access publication of this volume has received part funding via a Jane and Aatos Erkko Foundation grant. -

Kumpula Campus Code of Conduct � David J
Kumpula Campus Code of Conduct David J. Weir (he/him/his) Department of Physics - davidjamesweir This talk: saoghal.net/slides/code 14 March 2019 1 Outlook Kumpula Code of Conduct: Story Need Purpose Basis Contents Implementation 2 Story October 2018: Preparations begin within Physics Wellbeing Group. Support from Dean, Faculty Council and HR February 2019: Code of Conduct published First and only Code of Conduct at the University (so far) 3 Why do we need one? 4 Why do we need one? Helsinki's rules, values and policies are: Hidden Complicated In Flamma 4 Why do we need one? Helsinki's rules, values and policies are: Hidden Complicated In Flamma The Faculty of Science: Is an international and diverse community Has challenges in workplace wellbeing (stress, burnout, etc.) Actively tries to improve workplace wellbeing Has a history of cases of harassment Is active against harassment 4 Purpose Sets out clearly and positively: How we behave in the Faculty of Science. How we represent the Faculty of Science to the world. Behaviour we expect of each other and external partners. How we ensure that the Faculty is a safe place of work and study for everyone. Goal: everyone should feel welcome in our faculty. 5 What it is not It is not a trap – it should be common decency It is not a substitute for proper procedures – formal actions will always be taken when necessary It is not set in stone – society + university will change, the code will change too 6 Basis Values of the University of Helsinki (we had to start somewhere) University Strategy 2017-2020 [PDF on Flamma] Key inspiration: CERN Code of Conduct 7 Truth and knowledge We are guided in our actions by our core values of truth and knowledge, autonomy, creativity, critical mind, edification and wellbeing. -

In Words and Images
IN WORDS AND IMAGES 2017 Table of Contents 3 Introduction 4 The Academy Is the Academy 50 Estonia as a Source of Inspiration Is the Academy... 5 Its Ponderous Birth 52 Other Bits About Us 6 Its Framework 7 Two Pictures from the Past 52 Top of the World 55 Member Ene Ergma Received a Lifetime Achievement Award for Science 12 About the fragility of truth Communication in the dialogue of science 56 Academy Member Maarja Kruusmaa, Friend and society of Science Journalists and Owl Prize Winner 57 Friend of the Press Award 57 Six small steps 14 The Routine 15 The Annual General Assembly of 19 April 2017 60 Odds and Ends 15 The Academy’s Image is Changing 60 Science Mornings and Afternoons 17 Cornelius Hasselblatt: Kalevipoja sõnum 61 Academy Members at the Postimees Meet-up 20 General Assembly Meeting, 6 December 2017 and at the Nature Cafe 20 Fresh Blood at the Academy 61 Academic Columns at Postimees 21 A Year of Accomplishments 62 New Associated Societies 22 National Research Awards 62 Stately Paintings for the Academy Halls 25 An Inseparable Part of the National Day 63 Varia 27 International Relations 64 Navigating the Minefield of Advising the 29 Researcher Exchange and Science Diplomacy State 30 The Journey to the Lindau Nobel Laureate 65 Europe “Mining” Advice from Academies Meetings of Science 31 Across the Globe 66 Big Initiatives Can Be Controversial 33 Ethics and Good Practices 34 Research Professorship 37 Estonian Academy of Sciences Foundation 38 New Beginnings 38 Endel Lippmaa Memorial Lecture and Memorial Medal 40 Estonian Young Academy of Sciences 44 Three-minute Science 46 For Women in Science 46 Appreciation of Student Research Efforts 48 Student Research Papers’ π-prizes Introduction ife in the Academy has many faces. -

Second World War As a Trigger for Transcultural Changes Among Sámi People in Finland
Acta Borealia A Nordic Journal of Circumpolar Societies ISSN: 0800-3831 (Print) 1503-111X (Online) Journal homepage: http://www.tandfonline.com/loi/sabo20 Second world war as a trigger for transcultural changes among Sámi people in Finland Veli-Pekka Lehtola To cite this article: Veli-Pekka Lehtola (2015) Second world war as a trigger for transcultural changes among Sámi people in Finland, Acta Borealia, 32:2, 125-147, DOI: 10.1080/08003831.2015.1089673 To link to this article: http://dx.doi.org/10.1080/08003831.2015.1089673 Published online: 07 Oct 2015. Submit your article to this journal Article views: 22 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=sabo20 Download by: [Oulu University Library] Date: 23 November 2015, At: 04:24 ACTA BOREALIA, 2015 VOL. 32, NO. 2, 125–147 http://dx.doi.org/10.1080/08003831.2015.1089673 Second world war as a trigger for transcultural changes among Sámi people in Finland Veli-Pekka Lehtola Giellagas Institute, University of Oulu, Oulu, Finland ABSTRACT ARTICLE HISTORY The article analyses the consequences of the Lapland War (1944– Received 28 October 2014 45) and the reconstruction period (1945–52) for the Sámi society Revised 25 February 2015 in Finnish Lapland, and provides some comparisons to the Accepted 24 July 2015 situation in Norway. Reconstructing the devastated Lapland KEYWORDS meant powerful and rapid changes that ranged from novelties Sámi history; Finnish Lapland; of material culture to increasing Finnish ideals, from a Lapland War; reconstruction transition in the way of life to an assimilation process. -

Club Health Assessment for District 107 a Through September 2020
Club Health Assessment for District 107 A through September 2020 Status Membership Reports Finance LCIF Current YTD YTD YTD YTD Member Avg. length Months Yrs. Since Months Donations Member Members Members Net Net Count 12 of service Since Last President Vice Since Last for current Club Club Charter Count Added Dropped Growth Growth% Months for dropped Last Officer Rotation President Activity Account Fiscal Number Name Date Ago members MMR *** Report Reported Report *** Balance Year **** Number of times If below If net loss If no When Number Notes the If no report on status quo 15 is greater report in 3 more than of officers thatin 12 months within last members than 20% months one year repeat do not haveappears in two years appears appears appears in appears in terms an active red Clubs less than two years old 137239 Åland Culinaria 02/11/2019 Active 27 0 0 0 0.00% 20 3 M,VP,MC,SC N/R 90+ Days 142292 Turku Sirius 07/22/2020 Newly 25 26 1 25 100.00% 0 0 0 N 0 $200.00 Chartered Clubs more than two years old 119850 ÅBO/SKOLAN 06/27/2013 Cancelled(6*) 0 0 0 0 0.00% 5 1 1 None P,S,T,M,VP 24+ MC,SC M,MC,SC 59671 ÅLAND/FREJA 06/03/1997 Active 33 1 1 0 0.00% 32 4 0 N 16 Exc Award (06/30/2019) M,MC,SC 41195 ÅLAND/SÖDRA 04/14/1982 Active 28 0 0 0 0.00% 30 4 N 15 Exc Award (06/30/2019) 20334 AURA 11/07/1968 Active 38 0 0 0 0.00% 42 1 2 N SC 6 98864 AURA/SISU 03/22/2007 Active 20 2 1 1 5.26% 20 10 0 N 0 50840 BRÄNDÖ-KUMLINGE 07/03/1990 Active 15 0 0 0 0.00% 15 0 N M,MC,SC 9 32231 DRAGSFJÄRD 05/05/1976 Active 20 0 2 -2 -9.09% 24 9 0 N MC,SC 24+ 20339 -

Ecological and Chemical Aspects of White Oak Decline and Sudden Oak Death, Two Syndromes Associated with Phytophthora Spp
ECOLOGICAL AND CHEMICAL ASPECTS OF WHITE OAK DECLINE AND SUDDEN OAK DEATH, TWO SYNDROMES ASSOCIATED WITH PHYTOPHTHORA SPP. A Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Graduate School of The Ohio State University By Annemarie Margaret Nagle Graduate Program in Plant Pathology The Ohio State University 2009 *** Thesis Committee: Pierluigi (Enrico) Bonello, Advisor Laurence V. Madden Robert P. Long Dennis J. Lewandowski Copyright by: Annemarie Margaret Nagle 2009 ABSTRACT Phytophthora spp., especially invasives, are endangering forests globally. P. ramorum causes lethal canker diseases on coast live oak (CLO) and tanoak, and inoculation studies have demonstrated pathogenicity on other North American oak species, particularly those in the red oak group such as northern red oak (NRO). No practical controls are available for this disease, and characterization of natural resistance is highly desirable. Variation in resistance to P. ramorum has been observed in CLO in both naturally infected trees and controlled inoculations. Previous studies suggested that phloem phenolic chemistry may play a role in induced defense responses to P. ramorum in CLO (Ockels et al. 2007) but did not establish a relationship between these defense responses and actual resistance. Here we describe investigations aiming to elucidate the role of constitutive phenolics in resistance by quantifying relationships between concentrations of individual compounds, total phenolics, and actual resistance in CLO and NRO. Four experiments were conducted. In the first, we used cohorts of CLOs previously characterized as relatively resistant (R) or susceptible (S). Constitutive (pre-inoculation) phenolics were extracted from R and S branches on three different dates. -

Pasvik–Inari Nature and History Shared Area Description
PASVIK–INARI NATURE AND HISTORY SHARED AREA DESCRIPTION The Pasvik River flows from the largest lake in Finn- is recommended only for very experienced hikers, ish Lapland, Lake Inari, and extends to the Barents some paths are marked for shorter visits. Lake Inari Sea on the border of Norway and Russia. The valley and its tributaries are ideal for boating or paddling, forms a diverse habitat for a wide variety of plants and in winter the area can be explored on skis or a and animals. The Pasvik River is especially known for dog sled. The border mark at Muotkavaara, where its rich bird life. the borders of Finland, Norway and Russia meet, can The rugged wilderness that surrounds the river be reached by foot or on skis. valley astonishes with its serene beauty. A vast Several protected areas in the three neighbouring pine forest area dotted with small bogs, ponds and countries have been established to preserve these streams stretches from Vätsäri in Finland to Pasvik in great wilderness areas. A vast trilateral co-operation Norway and Russia. area stretching across three national borders, con- The captivating wilderness offers an excellent sisting of the Vätsäri Wilderness Area in Finland, the setting for hiking and recreation. From mid-May Øvre Pasvik National Park, Øvre Pasvik Landscape until the end of July the midnight sun lights up the Protection Area and Pasvik Nature Reserve in Nor- forest. The numerous streams and lakes provide way, and Pasvik Zapovednik in Russia, is protected. ample catch for anglers who wish to enjoy the calm backwoods. -

Laura Stark Peasants, Pilgrims, and Sacred Promises Ritual and the Supernatural in Orthodox Karelian Folk Religion
laura stark Peasants, Pilgrims, and Sacred Promises Ritual and the Supernatural in Orthodox Karelian Folk Religion Studia Fennica Folkloristica The Finnish Literature Society (SKS) was founded in 1831 and has, from the very beginning, engaged in publishing operations. It nowadays publishes literature in the fields of ethnology and folkloristics, linguistics, literary research and cultural history. The first volume of the Studia Fennica series appeared in 1933. Since 1992, the series has been divided into three thematic subseries: Ethnologica, Folkloristica and Linguistica. Two additional subseries were formed in 2002, Historica and Litteraria. The subseries Anthropologica was formed in 2007. In addition to its publishing activities, the Finnish Literature Society maintains research activities and infrastructures, an archive containing folklore and literary collections, a research library and promotes Finnish literature abroad. Studia fennica editorial board Anna-Leena Siikala Rauno Endén Teppo Korhonen Pentti Leino Auli Viikari Kristiina Näyhö Editorial Office SKS P.O. Box 259 FI-00171 Helsinki www.finlit.fi Laura Stark Peasants, Pilgrims, and Sacred Promises Ritual and the Supernatural in Orthodox Karelian Folk Religion Finnish Literature Society • Helsinki 3 Studia Fennica Folkloristica 11 The publication has undergone a peer review. The open access publication of this volume has received part funding via Helsinki University Library. © 2002 Laura Stark and SKS License CC-BY-NC-ND 4.0 International. A digital edition of a printed book first published in 2002 by the Finnish Literature Society. Cover Design: Timo Numminen EPUB: eLibris Media Oy ISBN 978-951-746-366-9 (Print) ISBN 978-951-746-578-6 (PDF) ISBN 978-952-222-766-9 (EPUB) ISSN 0085-6835 (Studia Fennica) ISSN 1235-1946 (Studia Fennica Folkloristica) DOI: http://dx.doi.org/10.21435/sff.11 This work is licensed under a Creative Commons CC-BY-NC-ND 4.0 International License.