DRAFT April 2005
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review of Succimer for Treatment of Lead Poisoning
Review of Succimer for treatment of lead poisoning Glyn N Volans MD, BSc, FRCP. Department of Clinical Pharmacology, School of Medicine at Guy's, King's College & St Thomas' Hospitals, St Thomas' Hospital, London, UK Lakshman Karalliedde MB BS, DA, FRCA Consultant Medical Toxicologist, CHaPD (London), Health Protection Agency UK, Visiting Senior Lecturer, Division of Public Health Sciences, King's College Medical School, King's College , London Senior Research Collaborator, South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, Peradeniya, Sri Lanka. Heather M Wiseman BSc MSc Medical Toxicology Information Services, Guy’s and St Thomas’ NHS Foundation Trust, London SE1 9RT, UK. Contact details: Heather Wiseman Medical Toxicology Information Services Guy’s & St Thomas’ NHS Foundation Trust Mary Sheridan House Guy’s Hospital Great Maze Pond London SE1 9RT Tel 020 7188 7188 extn 51699 or 020 7188 0600 (admin office) Date 10th March 2010 succimer V 29 Nov 10.doc last saved: 29-Nov-10 11:30 Page 1 of 50 CONTENTS 1 Summary 2. Name of the focal point in WHO submitting or supporting the application 3. Name of the organization(s) consulted and/or supporting the application 4. International Nonproprietary Name (INN, generic name) of the medicine 5. Formulation proposed for inclusion 6. International availability 7. Whether listing is requested as an individual medicine or as an example of a therapeutic group 8. Public health relevance 8.1 Epidemiological information on burden of disease due to lead poisoning 8.2 Assessment of current use 8.2.1 Treatment of children with lead poisoning 8.2.2 Other indications 9. -

Unit One Part 2: Naming and Functional Groups
gjr-–- 1 Unit One Part 2: naming and functional groups • To write and interpret IUPAC names for small, simple molecules • Identify some common functional groups found in organic molecules O CH3 H3C N H N O N N O H3C S N O N H3C viagra™ (trade name) sildenafil (trivial name) 5-(2-ethoxy-5-(4-methylpiperazin-1-ylsulfonyl)phenyl)-1-methyl-3-propyl-1H- pyrazolo[4,3-d] pyrimidin-7(6H)-one dr gareth rowlands; [email protected]; science tower a4.12 http://www.massey.ac.nz/~gjrowlan gjr-–- 2 Systematic (IUPAC) naming PREFIX PARENT SUFFIX substituents / minor number of C principal functional functional groups AND multiple bond group index • Comprises of three main parts • Note: multiple bond index is always incorporated in parent section No. Carbons Root No. Carbons Root Multiple-bond 1 meth 6 hex Bond index 2 eth 7 hept C–C an(e) 3 prop 8 oct C=C en(e) 4 but 9 non C≡C yn(e) 5 pent 10 dec gjr-–- 3 Systematic (IUPAC) naming: functional groups Functional group Structure Suffix Prefix General form O –oic acid acid –carboxylic acid carboxy R-COOH R OH O O –oic anhydride anhydride –carboxylic anhydride R-C(O)OC(O)-R R O R O –oyl chloride acyl chloride -carbonyl chloride chlorocarbonyl R-COCl R Cl O –oate ester –carboxylate alkoxycarbonyl R-COOR R OR O –amide amide –carboxamide carbamoyl R-CONH2 R NH2 nitrile R N –nitrile cyano R-C≡N O –al aldehyde –carbaldehyde oxo R-CHO R H O ketone –one oxo R-CO-R R R alcohol R OH –ol hydroxy R-OH amine R NH2 –amine amino R-NH2 O ether R R –ether alkoxy R-O-R alkyl bromide bromo R-Br (alkyl halide) R Br (halo) (R-X) gjr-–- 4 Nomenclature rules 1. -

Polymeric Reagents with Propane-1,3-Dithiol Functions and Their Precursors for Supported Organic Syntheses
J. Org. Chem. 2000, 65, 4839-4842 4839 Polymeric Reagents with Propane-1,3-dithiol Functions and Their Precursors for Supported Organic Syntheses Vincenzo Bertini,*,† Francesco Lucchesini,† Marco Pocci,† and Angela De Munno‡ Dipartimento di Chimica e Tecnologie Farmaceutiche ed Alimentari, Universita´ di Genova, Via Brigata Salerno, I-16147 Genova, Italy, and Dipartimento di Chimica e Chimica Industriale, Universita´ di Pisa, Via Risorgimento, 35, I-56126 Pisa, Italy [email protected] Received January 19, 2000 Reliable completely odorless syntheses of soluble copolymeric reagents of styrene type containing propane-1,3-dithiol functions able to convert carbonyl compounds into 1,3-dithiane derivatives and to support other useful transformations are reported together with their progenitor copolymers containing benzenesulfonate or thioacetate groups perfectly stable in open air and suitable for unlimited storage. The effectiveness of the prepared reagents as tools for polymer-supported syntheses to produce ketones by aldehyde umpolung and alkylation is tested in the conversion of benzaldehyde to phenyl n-hexyl ketone starting from copolymers with different contents of active units and molecular weights. To facilitate the adaptation of the prepared soluble copolymeric reagents to other possible applications, a table of solvents and nonsolvents is presented. Introduction Scheme 1 Recently we have reported in a preliminary com- munication1 how to harness the great synthetic potenti- alities of thioacetals and thioketals by preparing, with a completely odorless synthesis, polymeric reagents2,3 of styrene type containing propane-1,3-dithiol functions effective in producing ketones by aldehyde umpolung and alkylation after the formation of 1,3-dithiane derivatives. Such polymeric reagents were obtained through a pro- cedure based on the use of the difunctional monomer 1 prepared from commercial 4-vinylbenzyl chloride (Scheme 1), its copolymerization with styrene, and chemical modification of its copolymers. -

WIIINIPEG, I{ANI1.Oba October 1971
THE UNTVERSITY OF FJAI,TTTOBA STTIDIES ON TSOTHTÁ.ZOIIU}.I SÂLTS AND REIATED CO},IPOUI{DS by GART ED}¡ARD BACIIERS A T}MSIS SIMÌ,1TTTED TO TI{E FACULTY OF GR]I.DUÀTE STIIDIES ÏN PIIRÎI/iL FULIi'TIJ'&Til'r OF TI# REQUTREI.Tiü{TS TÇR TI.III DEGREE I'IÂSTER 0F SCIEÎIICE DEPARTI'ÎE]VT 0F CI{EI,IISTRY WIIINIPEG, I{ANI1.oBA October 1971 üF fi "*i¡l,''ÉËR$$iY LT BRARY ACIOTOIILEDGH,ÍTÍüTS : The research degcribed in thís thesis has been undertaken at tho suggestion of Dr. D. Id. McKínnon, for v¡hose guidance, advice, æd ass*stance ï exprees r¡\y sincere appreciation" My gratitud.e ie also due the National Research CounciÌ of Canada for the scholarships provid.ed. d.uring the course of this research, and the University of }ianitoba for the provisíon of laboratory facilities. Finally, I should like to thank rny colleaguee, I'fiss Sheena Loosmore, Ivlr. Joe Buchsbriber, Iilr. Pat Ma, a¡rd. Mr. Jack Wong. Their forbearance during several unusually od.or-ous experiments was admirable. 11 TA3I,E 0F.coNTlNTS ABSTRACT.......... o.. ó..... o. ...:. r. o õ i.. o.... o.......iii INTRODUCTION Ggngial.. - - . ô. :.' ..... o. ... .. .. .. .. r. .. o. .. .... 1 fsothiazoles. o r . c o. o . ... .. o. ¡. 2 ' I s o thi azo 1 um s s i al t . c . o . c . , . , . , o . ! 4-rsothiazoline-3-thiones... o... ô........ o.... r........ 13 . 3-Isothiazoline-l-thiones....o.....,r........er........18 Thiothiophthenes and an aza arialogue.. o...... c o. o... .,.19 DISCUSSION Purpose of resgarch...... c.. o...^... r.............. o., o..2J Reaction of isothiazolium salts ç¡ittr sutf:p¡ ¡ o o. -

WO 2014/195872 Al 11 December 2014 (11.12.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/195872 Al 11 December 2014 (11.12.2014) P O P C T (51) International Patent Classification: (74) Agents: CHOTIA, Meenakshi et al; K&S Partners | Intel A 25/12 (2006.01) A61K 8/11 (2006.01) lectual Property Attorneys, 4121/B, 6th Cross, 19A Main, A 25/34 (2006.01) A61K 8/49 (2006.01) HAL II Stage (Extension), Bangalore 560038 (IN). A01N 37/06 (2006.01) A61Q 5/00 (2006.01) (81) Designated States (unless otherwise indicated, for every A O 43/12 (2006.01) A61K 31/44 (2006.01) kind of national protection available): AE, AG, AL, AM, AO 43/40 (2006.01) A61Q 19/00 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A01N 57/12 (2006.01) A61K 9/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, AOm 59/16 (2006.01) A61K 31/496 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/IB20 14/06 1925 KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 3 June 2014 (03.06.2014) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (25) Filing Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Chelating Drug Therapy: an Update
Open Access Austin Journal of Genetics and Genomic Research Review Article Chelating Drug Therapy: An Update Vijay Kumar1, Ashok Kumar2*, Sandeep Kumar Singh1, Manoj Kumar3, Surendra Kumar2, Dinesh Abstract 4 5 Kumar and Ragni Singh Purpose: To study the clinical effects of metal toxicity and current 1Department of Neurology, SGPGIMS, India recommendations for management, including chelation therapy, are reviewed. 2Department of Medical Genetics, SGPGIMS, India 3Department of Microbiology, SGPGIMS, India Summary: Metals are essential to many biological processes, but excess 4Department of Chemistry, Dr. R.M.L. Avadh University, of it becomes hazardous to life. These are necessary for cell growth, electron India transport chain, several enzymatic activities and response of immune systems. 5Bheem Rao Ambedkar Bihar University, India They also serve as a cofactor for several enzymes. Chelation therapy is used for clinical management of the excess of metal. However, each metal requires *Corresponding author: Ashok Kumar, Department a specific chelation agent. A chelate is a compound form between metal and a of Medical Genetics, Sanjay Gandhi Post Graduate compound that contains two or more potential ligands. A promising Fe chelator Institute of Medical Sciences, Lucknow, India is Desferrioxamine (Desferal). Penicillamine and Trientine are uses for copper Received: March 12, 2015; Accepted: April 24, 2015; chelation. Meso-2,3-Dimercaptosuccinic Acid (DMSA) and 2,3-Dimercapto- Published: April 27, 2015 Propanesulphonate (DMPS) can be used as effective chelator of mercury. Dimercaprol, edetate calcium disodium, and succimer are the three agents primarily used for chelation of lead. Conclusion: Metal toxicity remains a significant public health concern. Elimination of elevated metal ions can be achieved by proper chelation agents. -

Changes in Tissue Gadolinium Biodistribution Measured in an Animal Model Exposed to Four Chelating Agents
Japanese Journal of Radiology (2019) 37:458–465 https://doi.org/10.1007/s11604-019-00835-1 ORIGINAL ARTICLE Changes in tissue gadolinium biodistribution measured in an animal model exposed to four chelating agents Türker Acar1,6 · Egemen Kaya2 · Mustafa Deniz Yoruk3 · Neslihan Duzenli4 · Recep Selim Senturk4 · Cenk Can4 · Lokman Ozturk3 · Canberk Tomruk5 · Yigit Uyanikgil5 · Frank J. Rybicki6 Received: 17 December 2018 / Accepted: 22 March 2019 / Published online: 30 March 2019 © Japan Radiological Society 2019 Abstract Purpose This study investigated the potential to reduce gadolinium levels in rodents after repetitive IV Gadodiamide admin- istration using several chelating agents. Materials and methods The following six groups of rats were studied. Group 1: Control; Group 2: Gadodiamide only; Group 3: Meso-2,3-Dimercaptosuccinic acid (DMSA) + Gadodiamide; Group 4: N-Acetyl-L-cysteine (NAC) + Gadodiamide; Group 5: Coriandrum sativum extract + Gadodiamide; and Group 6: Deferoxamine + Gadodiamide. Brain, kidney, and blood samples were evaluated via inductively coupled plasma mass spectrometry. The brain was also evaluated histologically. Results Kidney gadolinium levels in Groups 4 and 5 were approximately double that of Group 2 (p = 0.033 for each). There was almost no calcifcation in rat hippocampus for Group 4 rodents when compared with Groups 2, 3, 5 and 6. Conclusion Our preliminary study shows that excretion to the kidney has a higher propensity in NAC and Coriandrum sati- vum groups. It may be possible to change the distribution of gadolinium by administrating several agents. NAC may lower Gadodiamide-induced mineralization in rat hippocampus. Keywords Gadolinium deposition · Dimercaptosuccinic acid · N-Acetyl-L-cysteine · Coriandrum sativum · Deferoxamine Introduction However, the safety profle of gadolinium-based agents [1] was questioned after the discovery of nephrogenic sys- Gadolinium-based contrast agents (GBCA) have been safely temic fbrosis [2], a scleroderma-like disease characterized used in diagnostic radiology since the 1980s. -
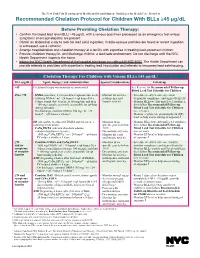
Recommended Chelation Protocol for Children with Blls ≥45 Μg/Dl
The New York City Department of Health and Mental Hygiene Guidelines for Health Care Providers Recommended Chelation Protocol for Children With BLLs ≥45 μg/dL Before Providing Chelation Therapy: • Confirm the blood lead level (BLL) ≥45 μg/dL with a venous specimen processed as an emergency test unless symptoms of encephalopathy are present. • Obtain an abdominal x-ray to look for lead solid ingestion; if radio-opaque particles are found or recent ingestion is witnessed, use a cathartic. • Arrange hospitalization and chelation therapy at a facility with expertise in treating lead-poisoned children. • Provide chelation therapy in, and discharge child to, a lead-safe environment. Do not discharge until the NYC Health Department inspects the home. • Inform the NYC Health Department of the hospital admission by calling 646-632-6002. The Health Department can provide referrals to providers with expertise in treating lead intoxication and referrals to temporary lead-safe housing. Chelation Therapy For Children with Venous BLLs ≥45 μg/dL1 BLL (μg/dL) Agent, Dosage,* and Administration Special Considerations Follow-up <45 Chelation therapy not routinely recommended See Reverse for Recommended Follow-up Blood Lead Test Schedule for Children 45 to <70 • DMSA (succimer, 2,3-meso-dimercaptosuccinic acid) • Monitor for anemia, • Schedule weekly health care visits • 1050 mg DMSA / m2 / 24 hours* ÷ q8 hours PO x neutropenia, and to monitor compliance and signs of toxicity. 5 days; round dose to nearest 100 mg/day, and then hepatic toxicity. • Monitor BLLs weekly until level stabilizes, ÷ 100-mg capsules as evenly as possible for q8-hour then follow Recommended Follow-up dosing schedule. -

Effect of Chromium(VI) on Serum Iron and Removal of Its Toxicity by Combining Deferasirox and Deferiprone Chelators in Rats
American Journal of Pharmacology and Toxicology 8 (4): 164-169, 2013 ISSN: 1557-4962 ©2013 Science Publication doi:10.3844/ajptsp.2013.164.169 Published Online 8 (4) 2013 (http://www.thescipub.com/ajpt.toc) Effect of Chromium(VI) on Serum Iron and Removal of its Toxicity by Combining Deferasirox and Deferiprone Chelators in Rats 1S. Jamil A. Fatemi, 1Marzieh Iranmanesh and 2Faezeh Dahooee Balooch 1Department of Chemistry, Faculty of Sciences, Islamic Azad University, Kerman Branch, Kerman, Iran 2Department of Chemistry, Faculty of Sciences, Shahid Bahonar University of Kerman, Kerman, Iran Received 2013-09-26, Revised 2013-10-21; Accepted 2013-10-30 ABSTRACT The present research is aimed to characterize the potential efficiency of two chelators after chromium(VI) administration for 60 days following two doses of 15 and 30 mg kg −1 chromium(VI) per body weight daily to male rats. However, the hypothesis that the two chelators might be more efficient as combined therapy than as single therapy in removing chromium(VI) from bood serum was considered. In this way, two known chelators deferasirox and deferiprone were chosen and tested in the acute rat model. Two chelators were given orally as a single or combined therapy for a period of one week. Chromium(VI) and iron concentrations in blood were determined by flame atomic absorption spectroscopy method. Chromium is one of the most widely used industrial metals. Several million workers worldwide are estimated to be exposed to chromium compounds in an array of industries. Chromium(VI) is more readily absorbed by both inhalation and oral routes. Ingestion of large amounts of chromium(VI) can lead to severe respiratory, cardiovascular, gastrointestinal, hepatic and renal damage and potentially death. -

Identification of a Common Chemical Signal Regulating the Induction Of
Proc. Nati. Acad. Sci. USA Vol. 85, pp. 8261-8265, November 1988 Medical Sciences Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis (Michael acceptors/quinone reductase/glutathione S-transferase/anticarcinogens) PAUL TALALAY, MARY J. DE LONG, AND HANS J. PROCHASKA Department of Pharmacology and Molecular Sciences, The Johns Hopkins University School of Medicine, Baltimore, MD 21205 Contributed by Paul Talalay, July 19, 1988 ABSTRACT Carcinogenesis is blocked by an extraordi- al inducers act are less clear (3). This paper extends our nary variety of agents belonging to many different classes- earlier efforts to identify structural features important for e.g., phenolic antioxidants, azo dyes, polycyclic aromatics, phase II enzyme induction by diphenols and phenylenedia- flavonoids, coumarins, cinnamates, indoles, isothiocyanates, mines (5). Since the specific activity of QR in the Hepa lclc7 1,2-dithiol-3-thiones, and thiocarbamates. The only known murine hepatoma cells is raised by virtually all compounds common property of these anticarcinogens is their ability to that produce coordinate elevations of phase II enzymes in elevate in animal cells the activities of enzymes that inactivate vivo (4, 5, 7), this system was used to determine the potency the reactive electrophilic forms of carcinogens. Structure- of various types of enzyme inducers. Some inducers identi- activity studies on the induction of quinone reductase fied in cell culture were also tested as inducers -

AX Pharmaceutical Corp Product List
AX Pharmaceutical Corp Product List A 4-Aminopyridine Acetazolamine ACTH 1-24 Acyclovir Sodium Adenosine 5 Monophosphate Adenosine 5 Tri-Phosphate Disodium Salt Albendazole Alendronate Sodium USP Alternogest Aminopentamide Sulfate Aminophylline Amlodipine Besylate Amoxicillin/Clavulanate Potassium 4:1 Amoxicillin Trihydrate Amphotericin B Ampicillin Anastrozole Aripepazole Aripiprazole Atipamezole HCl Atovaquone USP Atropine Sulfate Monohydrate Avanafil Azelastine HCl B Baclofen Benazepril HCl Betahistine Dihydrochloride Betaine Hydrochloride Betamethasone Acetate Betamethasone Dipropionate Betamethasone Sodium Phosphate Betaxolol Bexarotene Bicalutamide Bimatoprost Bisacodyl Bismuth Subcarbonate Bismuth Subsalicylate Bleomycin A5 Hydrochloride Bleomycin Sulfate Bretylium Tosylate Brimonidine Brinzolamide Bromhexine Bromocriptine Mesylate Brompheniramine Maleate Budesonide Bumetanide Bupivacaine Base Bupivacaine Hydrochloride Buprenorphine Bupropion HCl Buspirone Hydrochloride Busulfan Butaphosphan Butylated Hydroxyanisole C Cabergoline Calamine Calcium Glycerophosphate Calcium Levulinate Dihydrate Capsaicin Captopril Carbamazepine Carbazochrome Carbenoxolone Carbetocin Acetate Carbidopa Carbocisteine Carboplatin Carmustine Carprofen Carvedilol Cefadroxil Hemihydrate Cefadroxil Monohydrate Cefazolin Cefazolin Sodium Cefdinir Cefotaxime Cefotetan Disodium Cefpodoxime Cefpodoxime Proxetil Ceftazidime Ceftiofur Free Acid Ceftiofur Sodium Ceftriaxone Cefuroxime (Ceftin) Celecoxib Cephalexin Base Cephalexin Monohydrate Cesium Chloride Cetirizine -

514636-Unified-Chemistry.Pdf
Qualification Accredited A LEVEL Exemplar Candidate Work CHEMISTRY A H432 For first teaching in 2015 H432/03 Summer 2017 examination series Version 1 www.ocr.org.uk/chemistry A Level Chemistry A Exemplar Candidate Work Contents Introduction 3 Question 3(b) 20 Commentary 21 Question 1(a) 4 Commentary 4 Question 3(c)(i) 22 Commentary 22 Question 1(b) 5 Commentary 5 Question 3(c)(ii) 23 Commentary 24 Question 1(c) 6 Commentary 6 Question 4(a)(i) 25 Commentary 26 Question 1(d) 7 Commentary 7 Question 4(a)(ii) 27 Commentary 28 Question 1(e) 8 Commentary 8 Question 4(b)(i) 29 Commentary 29 Question 2(a) 9 Commentary 11 Question 4(b)(ii) 30 Commentary 30 Question 2(b) 12 Commentary 12 Question 4(c)(i) 31 Commentary 31 Question 2(c) 13 Commentary 13 Question 4(c)(ii) 32 Commentary 32 Question 2(d)(i) 14 Commenary 14 Question 5(a)(i) 33 Commentary 33 Question 2(d)(ii) 15 Commentary 15 Question 5(a)(ii) 34 Commentary 34 Question 2(d)(iii) 16 Commentary 16 Question 5(a)(iii) 35 Commentary 35 Question 3(a)(i) 17 Commentary 18 Question 5(a)(iv) 36 Commentary 36 Question 3(a)(ii) 19 Commentary 19 Question 5(b) 37 Commentary 38 2 © OCR 2017 A Level Chemistry A Exemplar Candidate Work Introduction These exemplar answers have been chosen from the summer 2017 examination series. OCR is open to a wide variety of approaches and all answers are considered on their merits. These exemplars, therefore, should not be seen as the only way to answer questions but do illustrate how the mark scheme has been applied.