Oogenesis: Single Cell Development and Differentiation ⁎ Jia L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
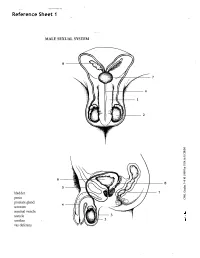
Reference Sheet 1
MALE SEXUAL SYSTEM 8 7 8 OJ 7 .£l"00\.....• ;:; ::>0\~ <Il '"~IQ)I"->. ~cru::>s ~ 6 5 bladder penis prostate gland 4 scrotum seminal vesicle testicle urethra vas deferens FEMALE SEXUAL SYSTEM 2 1 8 " \ 5 ... - ... j 4 labia \ ""\ bladderFallopian"k. "'"f"";".'''¥'&.tube\'WIT / I cervixt r r' \ \ clitorisurethrauterus 7 \ ~~ ;~f4f~ ~:iJ 3 ovaryvagina / ~ 2 / \ \\"- 9 6 adapted from F.L.A.S.H. Reproductive System Reference Sheet 3: GLOSSARY Anus – The opening in the buttocks from which bowel movements come when a person goes to the bathroom. It is part of the digestive system; it gets rid of body wastes. Buttocks – The medical word for a person’s “bottom” or “rear end.” Cervix – The opening of the uterus into the vagina. Circumcision – An operation to remove the foreskin from the penis. Cowper’s Glands – Glands on either side of the urethra that make a discharge which lines the urethra when a man gets an erection, making it less acid-like to protect the sperm. Clitoris – The part of the female genitals that’s full of nerves and becomes erect. It has a glans and a shaft like the penis, but only its glans is on the out side of the body, and it’s much smaller. Discharge – Liquid. Urine and semen are kinds of discharge, but the word is usually used to describe either the normal wetness of the vagina or the abnormal wetness that may come from an infection in the penis or vagina. Duct – Tube, the fallopian tubes may be called oviducts, because they are the path for an ovum. -

Homologous Repair of DNA Damage and Tumorigenesis: the BRCA Connection
Oncogene (2002) 21, 8981 – 8993 ª 2002 Nature Publishing Group All rights reserved 0950 – 9232/02 $25.00 www.nature.com/onc Homologous repair of DNA damage and tumorigenesis: the BRCA connection Maria Jasin*,1 1Cell Biology Program, Memorial Sloan-Kettering Cancer Center, New York, NY 10021, USA Homologous recombination has been recognized in recent Tirkkonen et al., 1997; Turner et al., 2002; Xu et al., years to be an important DNA repair pathway in 1999), are sensitive to DNA damaging agents (Bhatta- mammalian cells, for such damage as chromosomal charyya et al., 2000; Connor et al., 1997; Gowen et al., double-strand breaks. Cells mutated for the genes 1998; Kraakman-van der Zwet et al., 2002; Morimatsu involved in the hereditary breast and ovarian cancer et al., 1998; Moynahan et al., 2001a; Scully et al., 1999; susceptibility syndromes, i.e. BRCA1 and BRCA2, show Sharan et al., 1997; Shen et al., 1998; Zhong et al., defects in DNA repair by homologous recombination, 1999), including ionizing radiation and cross-linking implicating this repair pathway in protecting individuals agents, and have elevated mutation rates (Kraakman- against tumorigenesis. This review summarizes recent van der Zwet et al., 2002; Tutt et al., 2002). During S advances in our understanding of BRCA1 and BRCA2 in phase or upon treatment of cells with DNA damaging DNA repair, as well as insight into these proteins agents or replication inhibitors, both proteins are in gleaned from structure determination of domains of these nuclear foci along with several other proteins involved proteins and the broader evolutionary conservation than in the damage response, including the DNA repair previously appreciated. -

Effect of Paternal Age on Aneuploidy Rates in First Trimester Pregnancy Loss
Journal of Medical Genetics and Genomics Vol. 2(3), pp. 38-43, August 2010 Available online at http://www.academicjournals.org/jmgg ©2010 Academic Journals Full Length Research Paper Effect of paternal age on aneuploidy rates in first trimester pregnancy loss Vitaly A. Kushnir, Richard T. Scott and John L. Frattarelli 1Department of Obstetrics, Gynecology and Women’s Health, New Jersey Medical School, MSB E-506, 185 South Orange Avenue, Newark, NJ, 07101-1709, USA. 2Department of Obstetrics, Gynecology and Reproductive Sciences, Robert Wood Johnson Medical School UMDNJ, Division of Reproductive Endocrinology and Infertility, New Brunswick, NJ. Reproductive Medicine Associates of New Jersey, Morristown NJ, USA. Accepted 16 July, 2010 A retrospective cohort analysis of patients undergoing IVF cycles at an academic IVF center was performed to test the hypothesis that male age may influence aneuploidy rates in first trimester pregnancy losses. All patients had a first trimester pregnancy loss followed by evacuation of the pregnancy and karyotyping of the abortus. Couples undergoing anonymous donor oocyte ART cycles (n = 50) and 23 couples with female age less than 30 years undergoing autologous oocyte ART cycles were included. The oocyte age was less than 30 in both groups; thereby allowing the focus to be on the reproductive potential of the aging male. The main outcome measure was the effect of paternal age on aneuploidy rate. No increase in aneuploidy rate was noted with increasing paternal age (<40 years = 25.0%; 40-50 years = 38.8%; >50 years = 25.0%). Although there was a significant difference in the male partner age between oocyte recipients and young patients using autologous oocytes (33.7 7.6 vs. -

Spawning & Larviculture of Bivalve Mollusks
Spawning & Larviculture of Bivalve Mollusks Grade Level: Subject Area: Time: 9-12 Aquaculture, Biology, Preparation: 30 minutes to prepare Reproduction, Anatomy Activity: 50 minute lecture (may require 1 ½ class periods) Clean-up: NA SPS (SSS): 06.04 Employ correct terminologies for animal species and conditions (e.g. sex, age, etc.) (LA.A.1.4.1-4; LA.A.2.4.4; LA.B.1.4.1-3; LA.B.2.4.1-3; LA.C.1.4.1; LA.C.2.4.1). 11.09 Develop an information file in aquaculture species (LA.A.1.4, 2.4; LA.B.1.4, 2.4; LA.C.1.4, 2.4, 3.4; LA.D.2.4; SC.D.1.4; SC.F.1.4, 2.4; SC.G.1.4, 2.4). 11.10 List and describe the major factors in the growth of aquatic fauna and flora (LA.A.1.4, 2.4; LA.B.1.4, 2.4; LA.C.1.4, 2.4, 3.4; LA.D.2.4; SC.D.1.4, SC.F.1.4, 2.4; SC.G.1.4, 2.4). 13.02 Explain how changes in water affect aquatic life (LA.A.1.4, 2.4; LA.B.2.4; LA.C.1.4, 2.4; LA.D.2.4; SC.F.1.4; SC.G.1.4). 13.03 Explain, monitor, and maintain freshwater/saltwater quality standards for the production of desirable species (LA.A.1.4, 2.4; LA.B.2.4; LA.C.1.4, 2.4; LA.D.2.4; MA>B.1.4; MA.E.1.4, 2.4, 3.4; SC.E.2.4; SC.F.2.4; SC.G.1.4). -

Chapter 9 Reproduction in Animals.Pmd
9 Reproduction in Animals MULTIPLE CHOICE QUESTIONS 1. Sets of reproductive terms are given below. Choose the set that has an incorrect combination. (a) sperm, testis, sperm duct, penis (b) menstruation, egg, oviduct, uterus (c) sperm, oviduct, egg, uterus (d) ovulation, egg, oviduct, uterus 2. In humans, the development of fertilised egg takes place in the (a) ovary (c) oviduct (b) testis (d) uterus 3. In the list of animals given below, hen is the odd one out. human being, cow, dog, hen The reason for this is (a) it undergoes internal fertilisation. (b) it is oviparous. (c) it is viviparous. (d) it undergoes external fertilisation. 4. Animals exhibiting external fertilisation produce a large number of gametes. Pick the appropriate reason from the following. (a) The animals are small in size and want to produce more offsprings. (b) Food is available in plenty in water. (c) To ensure better chance of fertilisation. (d) Water promotes production of large number of gametes. 5. Reproduction by budding takes place in (a) hydra (c) paramecium (b) amoeba (d) bacteria 6. Which of the following statements about reproduction in humans is correct? (a) Fertilisation takes place externally. 12/04/18 48 EEE XEMPLAR PROBLEMS (b) Fertilisation takes place in the testes. (c) During fertilisation egg moves towards the sperm. (d) Fertilisation takes place in the human female. 7. In human beings, after fertilisation, the structure which gets embedded in the wall of uterus is (a) ovum (c) foetus (b) embryo (d) zygote 8. Aquatic animals in which fertilisation occurs in water are said to be: (a) viviparous without fertilisation. -

Gametogenesis: Spermatogenesis & Oogenesis -Structure of Sperm and Egg Fertilization
Gametogenesis: Spermatogenesis & Oogenesis ‐Structure of Sperm and Egg Fertilization ‐ Definition, Mechanism Early development in Frog ‐ Cleavage, Blas tu la, GtlGastrula, DitiDerivatives of Germ layers Vikasana - CET 2012 y Human reproduction y Brief Account of Fertilization: Implantation, Placenta, Role of Gonadotropins and sex hormones , Menstrual cycle. y Fertility Control: Family Planning Methods- y Infertility Control: Meaning, Causes,Treatment y STD: AIDS , Syphilis and Gonorrhea Vikasana - CET 2012 1.Primary Oocyte is a) Haploid (n) b) Diploid (2n) c) Polyploid d) None of the above Vikasana - CET 2012 2.Secondary Oocyte is a) Haploid (n) b) Diploid (2n) c) Polyploid d) None of the above Vikasana - CET 2012 3.Centrioles of sperm control a) Movement of tail b) Hap lo id numb er of ch romosomes c) Help in fertilization d) None of the above. Vikasana - CET 2012 4.The Fertilization membrane is secreted because a) It checks the entry of more sperms after fertilization b) it checks the entry of antigens in ovum c))p it represents the left out tail of the sperm d) it represen tVikasanas the p - l CETasma 2012 mem brane of the sperm 5.Meiosis I occurs in a) Primary spermatocytes b) Secondary spermatocytes c) Both a and b d) Spermatogonia Vikasana - CET 2012 6.Meiosis II occurs in a) Secondary oocyte b))y Primary oocyte c) Spermatogonia d) Oogonia Vikasana - CET 2012 7.Axial filament of sperm is formed by a) Distal centriole b) Prox ima l centitrio le c) Mitochondria d) DNA Vikasana - CET 2012 8.Polar bodies are formed during a) oogenesis -

The Role of Jelly Coats in Sperm-Egg Encounters, Fertilization Success, and Selection on Egg Size in Broadcast Spawners
vol. 157, no. 6 the american naturalist june 2001 The Role of Jelly Coats in Sperm-Egg Encounters, Fertilization Success, and Selection on Egg Size in Broadcast Spawners Gregory S. Farley* and Don R. Levitan† Department of Biological Science, Florida State University, 1996a, 1998b; Coma and Lasker 1997). The proposed mech- Tallahassee, Florida 32306-1100 anism generating this result is simply that larger egg targets are more likely to be struck by swimming sperm (Rothschild Submitted February 2, 2000; Accepted January 19, 2001 and Swann 1949, 1951; Vogel et al. 1982). This finding has generated the hypothesis that sperm availability can influ- ence the evolutionary trade-off between egg size and num- ber (Levitan 1993, 1996a, 1996b, 1998a, 1998b). However, abstract: Sperm limitation may be an important selective force the idea that fertilization kinetics might influence this trade- influencing gamete traits such as egg size. The relatively inexpensive off has been challenged because of the notion that extra- extracellular structures surrounding many marine invertebrate eggs might serve to enhance collision rates without the added cost of cellular structures or chemoattractants may increase sperm- increasing the egg cell. However, despite decades of research, the egg collisions yet may not represent as great a cost to effects of extracellular structures on fertilization have not been con- fecundity as the trade-off associated with increased egg size clusively documented. Here, using the sea urchin Lytechinus varie- (Podolsky and Strathmann 1996; Styan 1998). These con- gatus, we remove jelly coats from eggs, and we quantify sperm col- tradictory ideas have not been resolved because data de- lisions to eggs with jelly coats, eggs without jelly coats, and inert tailing the influence of extracellular materials on fertilization plastic beads. -

Anatomy of Male Reproductive System
Reproductive System Anatomy of Male Reproductive System Function: producing offspring Major Organs propagation of the species External Reproductive Organs !in terms of evolution penis and scrotum – the only reason all the other systems exist Internal Organs: only major system that doesn’t work continuously ! only activated at puberty these structures form continuous tube: unlike most other organisms on planet Testes ! mammals only reproduce sexually epididymus humans are dieocious vas deferens ! separate sexed (many animals are monoecious or ejaculatory duct hermaphrodites) urethra in penis th in 7 week of embryonic development genes are activated that trigger differentiation of gonads Accessory organs seminal vesicles prostate gland bulbourethral glands 1. Penis and Scrotum penis is transfer organ glans ! expanded head prepuce ! foreskin both have modified sebaceous glands that produce waxy secretion = smegma Human Anatomy & Physiology: Reproductive System; Ziser Lecture Notes, 2013.4 1 Human Anatomy & Physiology: Reproductive System; Ziser Lecture Notes, 2013.4 2 a. seminiferous tubules penis contains erectile tissues that surrounds (700’ of seminiferous tubules in testes) the urethra ! functions in spermatogenesis: ! fill with blood during sexual arousal formation and maturation of sperm cells corpus spongiosum (lower – surrounds urethra) passes along ventral side of penis and in cross section: encloses urethra seminiferous tubules appear roughly circular and contain germinal epithelium 2 coropora cavernosum (upper) (containing germ cells) and sustentacular on dorsal side (Sertoli) cells Sertoli cells protect germ cells and promote all contain numerous tiny blood sinuses their development = lacunae b. interstitial cells scrotum keeps testes at cooler temperature are scattered between the seminiferous tubules ! sperm can only be produced at several degrees below function in hormone secretion normal body temp !testosterone 2. -

(TEX) Genes: a Review Focused on Spermatogenesis and Male Fertility
Bellil et al. Basic and Clinical Andrology (2021) 31:9 https://doi.org/10.1186/s12610-021-00127-7 REVIEW ARTICLE Open Access Human testis-expressed (TEX) genes: a review focused on spermatogenesis and male fertility Hela Bellil1, Farah Ghieh2,3, Emeline Hermel2,3, Béatrice Mandon-Pepin2,3 and François Vialard1,2,3* Abstract Spermatogenesis is a complex process regulated by a multitude of genes. The identification and characterization of male-germ-cell-specific genes is crucial to understanding the mechanisms through which the cells develop. The term “TEX gene” was coined by Wang et al. (Nat Genet. 2001; 27: 422–6) after they used cDNA suppression subtractive hybridization (SSH) to identify new transcripts that were present only in purified mouse spermatogonia. TEX (Testis expressed) orthologues have been found in other vertebrates (mammals, birds, and reptiles), invertebrates, and yeasts. To date, 69 TEX genes have been described in different species and different tissues. To evaluate the expression of each TEX/tex gene, we compiled data from 7 different RNA-Seq mRNA databases in humans, and 4 in the mouse according to the expression atlas database. Various studies have highlighted a role for many of these genes in spermatogenesis. Here, we review current knowledge on the TEX genes and their roles in spermatogenesis and fertilization in humans and, comparatively, in other species (notably the mouse). As expected, TEX genes appear to have a major role in reproduction in general and in spermatogenesis in humans but also in all mammals such as the mouse. Most of them are expressed specifically or predominantly in the testis. -

Fertilization Selection on Egg and Jelly-Coat Size in the Sand Dollar Dendraster Excentricus
Evolution, 55(12), 2001, pp. 2479±2483 FERTILIZATION SELECTION ON EGG AND JELLY-COAT SIZE IN THE SAND DOLLAR DENDRASTER EXCENTRICUS DON R. LEVITAN1,2 AND STACEY D. IRVINE2 1Department of Biological Science, Florida State University, Tallahassee, Florida 32306-1100 2Bam®eld Marine Station, Bam®eld, British Columbia VOR 1B0, Canada Abstract. Organisms with external fertilization are often sperm limited, and in echinoids, larger eggs have a higher probability of fertilization than smaller eggs. This difference is thought to be a result of the more frequent sperm- egg collisions experienced by larger targets. Here we report how two components of egg target size, the egg cell and jelly coat, contributed to fertilization success in a selection experiment. We used a cross-sectional analysis of correlated characters to estimate the selection gradients on egg and jelly-coat size in ®ve replicate male pairs of the sand dollar Dendraster excentricus. Results indicated that eggs with larger cells and jelly coats were preferentially fertilized under sperm limitation in the laboratory. The selection gradients were an average of 922% steeper for egg than for jelly- coat size. The standardized selection gradients for egg and jelly-coat size were similar. Our results suggest that fertilization selection can act on both egg-cell and jelly-coat size but that an increase in egg-cell volume is much more likely to increase fertilization success than an equal change in jelly-coat volume. The strengths of the selection gradients were inversely related to the correlation of egg traits across replicate egg clutches. This result suggests the importance of replication in studies of selection of correlated characters. -
![Oogenesis [PDF]](https://docslib.b-cdn.net/cover/2902/oogenesis-pdf-452902.webp)
Oogenesis [PDF]
Oogenesis Dr Navneet Kumar Professor (Anatomy) K.G.M.U Dr NavneetKumar Professor Anatomy KGMU Lko Oogenesis • Development of ovum (oogenesis) • Maturation of follicle • Fate of ovum and follicle Dr NavneetKumar Professor Anatomy KGMU Lko Dr NavneetKumar Professor Anatomy KGMU Lko Oogenesis • Site – ovary • Duration – 7th week of embryo –primordial germ cells • -3rd month of fetus –oogonium • - two million primary oocyte • -7th month of fetus primary oocyte +primary follicle • - at birth primary oocyte with prophase of • 1st meiotic division • - 40 thousand primary oocyte in adult ovary • - 500 primary oocyte attain maturity • - oogenesis completed after fertilization Dr Navneet Kumar Dr NavneetKumar Professor Professor (Anatomy) Anatomy KGMU Lko K.G.M.U Development of ovum Oogonium(44XX) -In fetal ovary Primary oocyte (44XX) arrest till puberty in prophase of 1st phase meiotic division Secondary oocyte(22X)+Polar body(22X) 1st phase meiotic division completed at ovulation &enter in 2nd phase Ovum(22X)+polarbody(22X) After fertilization Dr NavneetKumar Professor Anatomy KGMU Lko Dr NavneetKumar Professor Anatomy KGMU Lko Dr Navneet Kumar Dr ProfessorNavneetKumar (Anatomy) Professor K.G.M.UAnatomy KGMU Lko Dr NavneetKumar Professor Anatomy KGMU Lko Maturation of follicle Dr NavneetKumar Professor Anatomy KGMU Lko Maturation of follicle Primordial follicle -Follicular cells Primary follicle -Zona pallucida -Granulosa cells Secondary follicle Antrum developed Ovarian /Graafian follicle - Theca interna &externa -Membrana granulosa -Antrial -

Egg White Foam
BAFFLING BEATERS Background Egg White Foam Egg white foam is a type of foam (a colloid in which a gas is dispersed or spread throughout a liquid) used in meringues, souffl és, and angel food cake to make them light and porous (airy). To prepare an egg white foam, egg whites are initially beaten (with a wire wisk or electric mixer) until they become frothy. Then an acid (such as cream of tartar) is added. Depending on the application, the beating of the egg white continues until soft (when the peaks stand straight and bend slightly at the tips) or stiff peaks (when the peaks stand straight without bending) are formed. Salt and sugar may also be added. How It Works: Egg whites are made up of water, protein, and small amounts of minerals and sugars. When the egg whites are beaten, air is added and the egg white protein, albumen, is denatured. Denaturation is the change of a protein’s shape under stress (in this case, beating). The denatured protein coats the air bubbles and holds in the water, causing them to References Food Mysteries Case 4: Protein Puzzlers. 1992. Originally developed by 4-H become stiff and stable. When an acid such as cream of tartar is added, Youth Development, Michigan State University Extension, East Lansing. the foam becomes even more stable and less likely to lose water (a process known as syneresis). Himich Freeland-Graves, J and Peckham, GC. 1996. Foundations of Food Preparation. 6th ed. Englewood Cliffs: Prentice Hall. 750 pgs. Several factors affect the formation and stability of egg white foams, including: • Fat: The addition of even a small amount of fat will interfere with the formation of a foam.