Rusty Patched Bumble Bee (Bombus Affinis) Ex Situ Assessment and Planning Workshop
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anthidium Manicatum, an Invasive Bee, Excludes a Native Bumble Bee, Bombus Impatiens, from floral Resources
Biol Invasions https://doi.org/10.1007/s10530-018-1889-7 (0123456789().,-volV)(0123456789().,-volV) ORIGINAL PAPER Anthidium manicatum, an invasive bee, excludes a native bumble bee, Bombus impatiens, from floral resources Kelsey K. Graham . Katherine Eaton . Isabel Obrien . Philip T. Starks Received: 15 April 2018 / Accepted: 21 November 2018 Ó Springer Nature Switzerland AG 2018 Abstract Anthidium manicatum is an invasive pol- response to A. manicatum presence. We found that B. linator reaching widespread distribution in North impatiens avoided foraging near A. manicatum in both America. Male A. manicatum aggressively defend years; but despite this resource exclusion, we found no floral territories, attacking heterospecific pollinators. evidence of fitness consequences for B. impatiens. Female A. manicatum are generalists, visiting many of These results suggest A. manicatum pose as significant the same plants as native pollinators. Because of A. resource competitors, but that B. impatiens are likely manicatum’s rapid range expansion, the territorial able to compensate for this resource loss by finding behavior of males, and the potential for female A. available resources elsewhere. manicatum to be significant resource competitors, invasive A. manicatum have been prioritized as a Keywords Exotic species Á Resource competition Á species of interest for impact assessment. But despite Interspecific competition Á Foraging behavior Á concerns, there have been no empirical studies inves- Pollination tigating the impact of A. manicatum on North Amer- ican pollinators. Therefore, across a two-year study, we monitored foraging behavior and fitness of the common eastern bumble bee (Bombus impatiens) in Introduction With increasing movement of goods and people Electronic supplementary material The online version of around the world, introduction of exotic species is this article (https://doi.org/10.1007/s10530-018-1889-7) con- increasing at an unprecedented rate (Ricciardi et al. -

Relationship of Insects to the Spread of Azalea Flower Spot
TECHNICAL BULLETIN NO. 798 • JANUARY 1942 Relationship of Insects to the Spread of Azalea Flower Spot By FLOYD F. SMITH Entomologist» Division of Truck Crop and Garden Insect Investigations Bureau of Entomology and Plant Quarantine and FREJEMAN WEISS Senior Pathologist, Division of Mycology and Disease Survey Bureau of Plant Industry UNITED STATES DEPARTMENT OF AGRICULTURE, WASHINGTON* D* C. For sale by the Superintendent of'Documents, Washington, D. G. • Price 10 cents Technical Bulletin No. 798 • January 1942 Relationship of Insects to the Spread of Azalea Flower Spot ^ By FLOYD F. SMITH, entomologist, Division of Truck Crop and Garden Insect Investigations, Bureau of Entomology and Plant Quarantine, and FREEMAN WEISS, senior pathologist. Division of Mycology and Disease Survey, Bureau of Plant Industry ^ CONTENTS Page Page Introduction ' 1 Disease transmission by insects II Insects visiting azaleas and observations on Preliminary studies, 1934 and 1935 11 their habits 2 Improved methods for collecting insects Bumblebees 2 and testing their infectivity 12 Carpenter bees 4 Studies in 1936 18 Ground-nesting bees 5 Transmission of flower spot on heads or Honeybees 5 legs or on pollen from insects- 20 Thrips 5 Transmission tests in 1937 and 1938 20 Ants 5 Relationship of insects to primary infection. 29 Flies 6 Other relationships of insects to the disease 33 Activity of bees in visiting flowers 6 Control experiments with insects on azaleas -. 39 Cause of insect abrasions and their relationship * E fîect of insecticid al dusts on bees 39 to flower spot infection < 7 Eiïect of poisoned sprays on bees 40 Occurrence on insects of conidia of the organ- Discussion of results 40 ism causing azalea flower spot 10 Summary 41 INTRODUCTION A serious spot disease and tlight was first reported in April 1931 near Charleston, S. -

Universidad Pol Facultad D Trabajo
UNIVERSIDAD POLITÉCNICA DE MADRID FACULTAD DE INFORMÁTICA TRABAJO FINAL DE CARRERA ESTUDIO DEL PROTOCOLO XMPP DE MESAJERÍA ISTATÁEA, DE SUS ATECEDETES, Y DE SUS APLICACIOES CIVILES Y MILITARES Autor: José Carlos Díaz García Tutor: Rafael Martínez Olalla Madrid, Septiembre de 2008 2 A mis padres, Francisco y Pilar, que me empujaron siempre a terminar esta licenciatura y que tanto me han enseñado sobre la vida A mis abuelos (q.e.p.d.) A mi hijo icolás, que me ha dejado terminar este trabajo a pesar de robarle su tiempo de juego conmigo Y muy en especial, a Susana, mi fiel y leal compañera, y la luz que ilumina mi camino Agradecimientos En primer lugar, me gustaría agradecer a toda mi familia la comprensión y confianza que me han dado, una vez más, para poder concluir definitivamente esta etapa de mi vida. Sin su apoyo, no lo hubiera hecho. En segundo lugar, quiero agradecer a mis amigos Rafa y Carmen, su interés e insistencia para que llegara este momento. Por sus consejos y por su amistad, les debo mi gratitud. Por otra parte, quiero agradecer a mis compañeros asesores militares de Nextel Engineering sus explicaciones y sabios consejos, que sin duda han sido muy oportunos para escribir el capítulo cuarto de este trabajo. Del mismo modo, agradecer a Pepe Hevia, arquitecto de software de Alhambra Eidos, los buenos ratos compartidos alrrededor de nuestros viejos proyectos sobre XMPP y que encendieron prodigiosamente la mecha de este proyecto. A Jaime y a Bernardo, del Ministerio de Defensa, por haberme hecho descubrir las bondades de XMPP. -
Download Windows Live Messenger for Linux Ubuntu
Download windows live messenger for linux ubuntu But installing applications in Ubuntu that were originally made for I found emescene to be the best Msn Messenger for Ubuntu Linux so far. It really gives you the feel as if you are using Windows Live Messenger. Its builds are available for Archlinux, Debian, Ubuntu, Fedora, Mandriva and Windows. At first I found it quite difficult to use Pidgin Internet Messenger on Ubuntu Linux. Even though it allows signing into MSN, Yahoo! Messenger and Google Talk. While finding MSN Messenger for Linux / Ubuntu, I found different emesene is also available and could be downloaded and installed for. At first I found it quite difficult to use Pidgin Internet Messenger on Ubuntu Linux. Even though it allows signing into MSN, Yahoo! Messenger. A simple & beautiful app for Facebook Messenger. OS X, Windows & Linux By downloading Messenger for Desktop, you acknowledge that it is not an. An alternative MSN Messenger chat client for Linux. It allows Linux users to chat with friends who use MSN Messenger in Windows or Mac OS. The strength of. Windows Live Messenger is an instant messenger application that For more information on installing applications, see InstallingSoftware. sudo apt-get install chromium-browser. 2. After the installation is Windows Live Messenger running in LinuxMint / Ubuntu. You can close the. Linux / X LAN Messenger for Debian/Ubuntu LAN Messenger for Fedora/openSUSE Download LAN Messenger for Windows. Windows installer A MSN Messenger / Live Messenger client for Linux, aiming at integration with the KDE desktop Ubuntu: Ubuntu has KMess in its default repositories. -
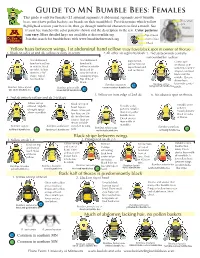
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

NIH Public Access Author Manuscript J Eukaryot Microbiol
NIH Public Access Author Manuscript J Eukaryot Microbiol. Author manuscript; available in PMC 2009 November 18. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Eukaryot Microbiol. 2009 ; 56(2): 142±147. doi:10.1111/j.1550-7408.2008.00374.x. Morphological, molecular, and phylogenetic characterization of Nosema ceranae, a microsporidian parasite isolated from the European honey bee, Apis mellifera Y.P. Chena, J.D. Evansa, C. Murphyb, R. Gutellc, M. Zukerd, D. Gundensen-Rindale, and J.S. Pettisa a USDA-ARS, Bee Research Laboratory, Beltsville, MD, USA b USDA-ARS, Soybean Genomic & Improvement Laboratory, Beltsville, MD, USA c Institute for Cellular and Molecular Biology and Section of Integrative Biology, University of Texas, Austin, TX, USA d Rensselaer Polytechnic Institute, NY, USA e USDA-ARS, Invasive Insect Biocontrol and Behavior Laboratory, Beltsville, MD, USA Abstract Nosema ceranae, a microsporidian parasite originally described from Apis cerana, has been found to infect Apis melllifera and is highly pathogenic to its new host. In the present study, data on the ultrastructure of N. ceranae, presence of N. ceranae-specific nucleic acid in host tissues, and phylogenetic relationships with other microsporidia species are described. The ultrastructural features indicate that N. ceranae possesses all of the characteristics of the genus Nosema. Spores of N. ceranae measured approximately 4.4 × 2.2 μm on fresh smears. The number of coils of the polar filament inside spores was 18--21. PCR signals specific for N. ceranae were detected not only in the primary infection site, the midgut, but also in the tissues of hypopharyngeal glands, salivary glands, Malpighian tubules, and fat body. -

Recursos Florales Usados Por Dos Especies De Bombus En Un Fragmento De Bosque Subandino (Pamplonita-Colombia)
ARTÍCULO ORIGINAL REVISTA COLOMBIANA DE CIENCIA ANIMAL Rev Colombiana Cienc Anim 2017; 9(1):31-37. Recursos florales usados por dos especies de Bombus en un fragmento de bosque subandino (Pamplonita-Colombia) Floral resources use by two species of Bombus in a subandean forest fragment (Pamplonita-Colombia) 1* 2 3 Mercado-G, Jorge M.Sc, Solano-R, Cristian Biol, Wolfgang R, Hoffmann Biol. 1Universidad de Sucre, Departamento de Biología y Química, Grupo Evolución y Sistemática Tropical. Sincelejo. Colombia. 2IANIGLA, Departamento de Paleontología, CCT CONICET. Mendoza, Argentina. 3Universidad de Pamplona, Facultad de Ciencias Básicas, Grupo de Biocalorimetría. Pamplona, Colombia Keywords: Abstract Pollen; It is present the results of a palynological analysis of two species of the genus palinology; Bombus in vicinity of Pamplonita-Norte of Santander. On feces samples were diet; identified and counted (april and may of 2010) 1585 grains of pollen, within which Norte de Santander; Solanaceae, Asteraceae, Malvaceae and Fabaceae were the more abundat taxa. Hymenoptera, feces. With regard to the diversity, April was the most rich, diverse and dominant month; however in B. pullatus we observed greater wealth and dominance. Finally, it was possible to determine that a large percentage of pollen grains that were identified as Solanaceae correspond to Solanum quitoense. The results of this study make clear the importance of palynology at the time of establishing the diet of these species, in addition to reflect its role of those species as possible provider in ecosistemic services either in wild plant as a crops in Solanaceae case. Palabras Clave: Resumen Polen; Se presentan los resultados de un análisis palinológico en dos especies del palinología; género Bombus en cercanías de Pamplonita (Norte de Santander-Colombia) dieta; durante los meses de abril y mayo de 2010. -

Species Knowledge Review: Shrill Carder Bee Bombus Sylvarum in England and Wales
Species Knowledge Review: Shrill carder bee Bombus sylvarum in England and Wales Editors: Sam Page, Richard Comont, Sinead Lynch, and Vicky Wilkins. Bombus sylvarum, Nashenden Down nature reserve, Rochester (Kent Wildlife Trust) (Photo credit: Dave Watson) Executive summary This report aims to pull together current knowledge of the Shrill carder bee Bombus sylvarum in the UK. It is a working document, with a view to this information being reviewed and added when needed (current version updated Oct 2019). Special thanks to the group of experts who have reviewed and commented on earlier versions of this report. Much of the current knowledge on Bombus sylvarum builds on extensive work carried out by the Bumblebee Working Group and Hymettus in the 1990s and early 2000s. Since then, there have been a few key studies such as genetic research by Ellis et al (2006), Stuart Connop’s PhD thesis (2007), and a series of CCW surveys and reports carried out across the Welsh populations between 2000 and 2013. Distribution and abundance Records indicate that the Shrill carder bee Bombus sylvarum was historically widespread across southern England and Welsh lowland and coastal regions, with more localised records in central and northern England. The second half of the 20th Century saw a major range retraction for the species, with a mixed picture post-2000. Metapopulations of B. sylvarum are now limited to five key areas across the UK: In England these are the Thames Estuary and Somerset; in South Wales these are the Gwent Levels, Kenfig–Port Talbot, and south Pembrokeshire. The Thames Estuary and Gwent Levels populations appear to be the largest and most abundant, whereas the Somerset population exists at a very low population density, the Kenfig population is small and restricted. -

The Conservation Management and Ecology of Northeastern North
THE CONSERVATION MANAGEMENT AND ECOLOGY OF NORTHEASTERN NORTH AMERICAN BUMBLE BEES AMANDA LICZNER A DISSERTATION SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY GRADUATE PROGRAM IN BIOLOGY YORK UNIVERSITY TORONTO, ONTARIO September 2020 © Amanda Liczner, 2020 ii Abstract Bumble bees (Bombus spp.; Apidae) are among the pollinators most in decline globally with a main cause being habitat loss. Habitat requirements for bumble bees are poorly understood presenting a research gap. The purpose of my dissertation is to characterize the habitat of bumble bees at different spatial scales using: a systematic literature review of bumble bee nesting and overwintering habitat globally (Chapter 1); surveys of local and landcover variables for two at-risk bumble bee species (Bombus terricola, and B. pensylvanicus) in southern Ontario (Chapter 2); identification of conservation priority areas for bumble bee species in Canada (Chapter 3); and an analysis of the methodology for locating bumble bee nests using detection dogs (Chapter 4). The main findings were current literature on bumble bee nesting and overwintering habitat is limited and biased towards the United Kingdom and agricultural habitats (Ch.1). Bumble bees overwinter underground, often on shaded banks or near trees. Nests were mostly underground and found in many landscapes (Ch.1). B. terricola and B. pensylvanicus have distinct habitat characteristics (Ch.2). Landscape predictors explained more variation in the species data than local or floral resources (Ch.2). Among local variables, floral resources were consistently important throughout the season (Ch.2). Most bumble bee conservation priority areas are in western Canada, southern Ontario, southern Quebec and across the Maritimes and are most often located within woody savannas (Ch.3). -

Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture
Matteson and Langellotto: URBAN BUMBLE BEE ABUNDANCE Cities and the Environment 2009 Volume 2, Issue 1 Article 5 Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture Kevin C. Matteson and Gail A. Langellotto Abstract A variety of crops are grown in New York City community gardens. Although the production of many crops benefits from pollination by bees, little is known about bee abundance in urban community gardens or which crops are specifically dependent on bee pollination. In 2005, we compiled a list of crop plants grown within 19 community gardens in New York City and classified these plants according to their dependence on bee pollination. In addition, using mark-recapture methods, we estimated the abundance of a potentially important pollinator within New York City urban gardens, the common eastern bumble bee (Bombus impatiens). This species is currently recognized as a valuable commercial pollinator of greenhouse crops. However, wild populations of B. impatiens are abundant throughout its range, including in New York City community gardens, where it is the most abundant native bee species present and where it has been observed visiting a variety of crop flowers. We conservatively counted 25 species of crop plants in 19 surveyed gardens. The literature suggests that 92% of these crops are dependent, to some degree, on bee pollination in order to set fruit or seed. Bombus impatiens workers were observed visiting flowers of 78% of these pollination-dependent crops. Estimates of the number of B. impatiens workers visiting individual gardens during the study period ranged from 3 to 15 bees per 100 m2 of total garden area and 6 to 29 bees per 100 m2 of garden floral area. -

The Rusty Patched Bumble Bee (Bombus Affinis) Voluntary Implementation Guidance for Section 10(A)(1)(B) of the Endangered Species Act
The Rusty Patched Bumble Bee (Bombus affinis) Voluntary Implementation Guidance for Section 10(a)(1)(B) of the Endangered Species Act Version 1.1 U.S. Fish & Wildlife Service, Regions 3, 4, 5 and 6 March 21, 2017 Contents Background and Purpose ................................................................................................................ 1 Current Versions of this Guidance .................................................................................................. 1 Range of Rusty Patched Bumble Bee .............................................................................................. 1 Brief Description of the Habitat Model ...................................................................................... 2 Section 10(a)(1)(B) of the Endangered Species Act and the Rusty Patched Bumble Bee .............. 5 Screening and Evaluation of Projects – A Stepwise Approach ................................................... 5 Step 1. Determine whether the rusty patched bumble bee is likely to be present in the project area. ............................................................................................................................ 5 Step 2 - Review the Project for its Potential to Incidentally Take the Species ....................... 8 Step 3 - Review Measures to Avoid Incidental Take of the Rusty Patched Bumble Bee ...... 14 Conservation Measures ............................................................................................................ 15 Restore and Maintain High Quality Habitat -

Global Trends in Bumble Bee Health
EN65CH11_Cameron ARjats.cls December 18, 2019 20:52 Annual Review of Entomology Global Trends in Bumble Bee Health Sydney A. Cameron1,∗ and Ben M. Sadd2 1Department of Entomology, University of Illinois, Urbana, Illinois 61801, USA; email: [email protected] 2School of Biological Sciences, Illinois State University, Normal, Illinois 61790, USA; email: [email protected] Annu. Rev. Entomol. 2020. 65:209–32 Keywords First published as a Review in Advance on Bombus, pollinator, status, decline, conservation, neonicotinoids, pathogens October 14, 2019 The Annual Review of Entomology is online at Abstract ento.annualreviews.org Bumble bees (Bombus) are unusually important pollinators, with approx- https://doi.org/10.1146/annurev-ento-011118- imately 260 wild species native to all biogeographic regions except sub- 111847 Saharan Africa, Australia, and New Zealand. As they are vitally important in Copyright © 2020 by Annual Reviews. natural ecosystems and to agricultural food production globally, the increase Annu. Rev. Entomol. 2020.65:209-232. Downloaded from www.annualreviews.org All rights reserved in reports of declining distribution and abundance over the past decade ∗ Corresponding author has led to an explosion of interest in bumble bee population decline. We Access provided by University of Illinois - Urbana Champaign on 02/11/20. For personal use only. summarize data on the threat status of wild bumble bee species across bio- geographic regions, underscoring regions lacking assessment data. Focusing on data-rich studies, we also synthesize recent research on potential causes of population declines. There is evidence that habitat loss, changing climate, pathogen transmission, invasion of nonnative species, and pesticides, oper- ating individually and in combination, negatively impact bumble bee health, and that effects may depend on species and locality.