Therapeutic Class Overview Immunomodulators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients
antibodies Review Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients Andrew T. Lucas 1,2,3,*, Ryan Robinson 3, Allison N. Schorzman 2, Joseph A. Piscitelli 1, Juan F. Razo 1 and William C. Zamboni 1,2,3 1 University of North Carolina (UNC), Eshelman School of Pharmacy, Chapel Hill, NC 27599, USA; [email protected] (J.A.P.); [email protected] (J.F.R.); [email protected] (W.C.Z.) 2 Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] 3 Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-919-966-5242; Fax: +1-919-966-5863 Received: 30 November 2018; Accepted: 22 December 2018; Published: 1 January 2019 Abstract: The rapid advancement in the development of therapeutic proteins, including monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), has created a novel mechanism to selectively deliver highly potent cytotoxic agents in the treatment of cancer. These agents provide numerous benefits compared to traditional small molecule drugs, though their clinical use still requires optimization. The pharmacology of mAbs/ADCs is complex and because ADCs are comprised of multiple components, individual agent characteristics and patient variables can affect their disposition. To further improve the clinical use and rational development of these agents, it is imperative to comprehend the complex mechanisms employed by antibody-based agents in traversing numerous biological barriers and how agent/patient factors affect tumor delivery, toxicities, efficacy, and ultimately, biodistribution. -

BCBSVT Specialty Drug List Effective 2021.07.01.Xlsx
Effective Date: 07/01/2021 SPECIALTY DRUG LIST Revised Date: 05/07/2021 DOSAGE EXCLUDED ON NATIONAL DRUG CLASS DRUG NAME GENERIC NAME FORM PERFORMANCE FORMULARY ANEMIA ARANESP SOLN DARBEPOETIN ALFA SOLN INJ ANEMIA ARANESP SOSY DARBEPOETIN ALFA SOLN PREFILLED SYRINGE ANEMIA EPOGEN SOLN EPOETIN ALFA INJ X ANEMIA PROCRIT SOLN EPOETIN ALFA INJ X ANEMIA REBLOZYL SOLR LUSPATERCEPT-AAMT FOR SUBCUTANEOUS INJ ANEMIA RETACRIT SOLN EPOETIN ALFA-EPBX INJ ANTI-GOUT AGENT KRYSTEXXA SOLN PEGLOTICASE INJ (FOR IV INFUSION) ANTI-INFECTIVE PREVYMIS SOLN LETERMOVIR IV SOLN ANTI-INFECTIVE PREVYMIS TABS LETERMOVIR TAB ASTHMA CINQAIR SOLN RESLIZUMAB IV INFUSION SOLN ASTHMA FASENRA SOSY BENRALIZUMAB SUBCUTANEOUS SOLN PREFILLED SYRINGE ASTHMA FASENRA PEN SOAJ BENRALIZUMAB SUBCUTANEOUS SOLN AUTO-INJECTOR ASTHMA NUCALA SOAJ MEPOLIZUMAB SUBCUTANEOUS SOLUTION AUTO-INJECTOR ASTHMA NUCALA SOLR MEPOLIZUMAB FOR INJ ASTHMA NUCALA SOSY MEPOLIZUMAB SUBCUTANEOUS SOLUTION PREF SYRINGE ASTHMA XOLAIR SOLR OMALIZUMAB FOR INJ ASTHMA XOLAIR SOSY OMALIZUMAB SUBCUTANEOUS SOLN PREFILLED SYRINGE CARDIOVASCULAR VYNDAMAX CAPS TAFAMIDIS CAP CARDIOVASCULAR VYNDAQEL CAPS TAFAMIDIS MEGLUMINE (CARDIAC) CAP CENTRAL NERVOUS SYSTEM AGENTS AUSTEDO TABS DEUTETRABENAZINE TAB CENTRAL NERVOUS SYSTEM AGENTS ENSPRYNG SOSY SATRALIZUMAB-MWGE SUBCUTANEOUS SOLN PREF SYRINGE CENTRAL NERVOUS SYSTEM AGENTS HETLIOZ CAPS TASIMELTEON CAPSULE CENTRAL NERVOUS SYSTEM AGENTS HETLIOZ LQ SUSP TASIMELTEON ORAL SUSP CHEMOTHERAPY PROTECTANT AMIFOSTINE SOLR AMIFOSTINE CRYSTALLINE FOR INJ CHEMOTHERAPY PROTECTANT ELITEK -

Inflammatory Conditions – Kevzara™ (Sarilumab for Subcutaneous Injection)
Cigna National Formulary Coverage Policy Prior Authorization Inflammatory Conditions – Kevzara® (sarilumab for subcutaneous injection) Table of Contents Product Identifier(s) National Formulary Medical Necessity ................ 1 59231 Conditions Not Covered....................................... 2 Background .......................................................... 2 References .......................................................... 3 Revision History ................................................... 3 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer’s benefit plan document always supersedes the information in the Coverage Policies. In the absence of a controlling federal or state coverage mandate, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific instance require consideration of 1) the terms of the applicable benefit plan document in effect on the date of service; 2) any applicable laws/regulations; 3) any relevant collateral source materials including Coverage Policies and; 4) the specific facts of the particular situation. -

New Biologics in Psoriasis: an Update on IL-23 and IL-17 Inhibitors
New Biologics in Psoriasis: An Update on IL-23 and IL-17 Inhibitors Joanna Dong, BA; Gary Goldenberg, MD PRACTICE POINTS • The newest biologics for treatment of moderate to severe plaque psoriasis are IL-23 and IL-17 inhibitors with unprecedented efficacy of complete skin clearance compared to older biologics. • Risankizumab, guselkumab, and tildrakizumab are new IL-23 inhibitors currently in phase 3 trials with promising early efficacy and safety results. • Ixekizumab, which recently was approved, and brodalumab, which is pending US Food and Drug Administration review, are new IL-17 inhibitors that achieved total skin clearance in more than one-quarter of phase 3 participants after 12 weeks of treatment. copy not As immune-related pathways involved in the he role of current biologic therapies in pso- pathogenesis of psoriasis are elucidated, new riasis predicates on the pathogenic role of biologic treatments targeting these steps of the Tupregulated, immune-related mechanisms psoriatic immune cascade are developed. In Dothis that result in the activation of myeloid dendritic article, we review the literature on IL-23 and IL-17 cells, which release IL-17, IL-23, and other cytokines inhibitors in the pipeline for use in moderate to to activate T cells, including helper T cell TH17. severe psoriasis. Numerous pipeline biologic Along with other immune cells, TH17 produces therapies, including risankizumab, guselkumab, IL-17. This proinflammatory cascade results in kera- tildrakizumab, ixekizumab, and brodalumab, are tinocyte proliferation, angiogenesis, and migration being investigated in phase 2 and 3 studies to of immune cells toward psoriatic lesions.1 Thus, the establish the efficacy and safety of these new newest classes of biologics target IL-12, IL-23, and agents. -

Emerging Therapies in Psoriasis: a Systematic Review
CLINICAL REVIEW Emerging Therapies In Psoriasis: A Systematic Review Erica B. Lee, BS; Mina Amin, BS; Tina Bhutani, MD; Jashin J. Wu, MD soriasis is a chronic, autoimmune-mediated disease PRACTICE POINTS estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a • Tumor necrosis factor α, I L-23, and IL-17A are key P targets for psoriasis therapy based on an under- complex process triggered by a combination of genetic standing of the key role that these cytokines play and environmental factors that induce tumor necrosis in the pathophysiology of disease. factor (TNF) α secretion by keratinocytes, which in turn • The biologic agents secukinumab and ixekizumab activates dendritic cells. Activated dendritic cells produce 2,3 are approved for use in the management of pso- IL-23, leading to helper T cell (TH17) differentiation. riasis. Other biologics—brodalumab, bimekizumab, TH17 cells secrete IL-17A, which has been shown to pro- guselkumab, tildrakizumab, risankizumab, and cer- mote psoriatic skincopy changes.4 Therefore, TNF-α, IL-23, tolizumab pegol—have been (and some continue to and IL-17A have been recognized as key targets for pso- be) the focus of phase 2 and phase 3 clinical trials. riasis therapy. • Findings of several of those trials support the idea that The newest biologic agents targeting IL-17– therapies targeting IL-23, specifically its p19 subunit, mediated pathways include ixekizumab, brodalumab, but that spare IL-12 are effective against psoriasis. andnot bimekizumab. Secukinumab, the first US Food and • Longer-term studies are needed to determine whether Drug Administration (FDA)–approved IL-17 inhibitor, the agents reviewed here, including those approved for has been available since 2015 and therefore is not clinical use, are suitable for prolonged administration. -

Immunfarmakológia Immunfarmakológia
Gergely: Immunfarmakológia Immunfarmakológia Prof Gergely Péter Az immunpatológiai betegségek döntő többsége gyulladásos, és ennek következtében általában szövetpusztulással járó betegség, melyben – jelenleg – a terápia alapvetően a gyulladás csökkentésére és/vagy megszűntetésére irányul. Vannak kizárólag gyulladásgátló gyógyszereink és vannak olyanok, amelyek az immunreakció(k) bénításával (=immunszuppresszió révén) vagy emellett vezetnek a gyulladás mérsékléséhez. Mind szerkezetileg, mind hatástanilag igen sokféle csoportba oszthatók, az alábbi felosztás elsősorban didaktikus célokat szolgál. 1. Nem-szteroid gyulladásgátlók (‘nonsteroidal antiinflammatory drugs’ NSAID) 2. Kortikoszteroidok 3. Allergia-elleni szerek (antiallergikumok) 4. Sejtoszlás-gátlók (citosztatikumok) 5. Nem citosztatikus hatású immunszuppresszív szerek 6. Egyéb gyulladásgátlók és immunmoduláns szerek 7. Biológiai terápia 1. Nem-szteroid gyulladásgátlók (NSAID) Ezeket a vegyületeket, melyek őse a szalicilsav (jelenleg, mint acetilszalicilsav ‘aszpirin’ használatos), igen kiterjedten alkalmazzák a reumatológiában, az onkológiában és az orvostudomány szinte minden ágában, ahol fájdalom- és lázcsillapításra van szükség. Egyes felmérések szerint a betegek egy ötöde szed valamilyen NSAID készítményt. Szerkezetük alapján a készítményeket több csoportba sorolhatjuk: szalicilátok (pl. acetilszalicilsav) pyrazolidinek (pl. fenilbutazon) ecetsav származékok (pl. indometacin) fenoxiecetsav származékok (pl. diclofenac, aceclofenac)) oxicamok (pl. piroxicam, meloxicam) propionsav -

761067Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 761067Orig1s000 RISK ASSESSMENT and RISK MITIGATION REVIEW(S) Division of Risk Management (DRISK) Office of Medication Error Prevention and Risk Management (OMEPRM) Office of Surveillance and Epidemiology (OSE) Center for Drug Evaluation and Research (CDER) Application Type BLA Application Number 761067 PDUFA Goal Date March 23, 2018 OSE RCM # 2017-605, 2017-606 Reviewer Name(s) Laura Zendel, PharmD, BCPS, Division of Risk Management (DRISK) Team Leader Donella Fitzgerald, PharmD, DRISK Deputy Director Jamie Wilkins Parker, PharmD, DRISK Review Completion Date March 7, 2018 Subject Evaluation of Need for a REMS Established Name Tildrakizumab Trade Name Ilumya Name of Applicant Merck Sharp & Dohme Corp. Therapeutic Class Human Interleukin 23 Antagonist Formulation(s) 100 mg/ml single-dose prefilled syringe Dosing Regimen 100mg administered by subcutaneous injection at week 0, week 4, and every 12 weeks thereafter. 1 Reference ID: 4231274 Table of Contents Executive Summary ................................................................................................................................................................ 3 1 Introduction ..................................................................................................................................................................... 3 2 Background ..................................................................................................................................................................... -

All Reviews for Use of Kevzara for COVID-19 And/Or Cytokine Release Syndrome Associated with COVID-19 Will Be Forwarded to the Medical Director
Policy: Kevzara™ (sarilumab) Annual Review Date: 08/20/2020 Last Revised Date: 08/20/2020 OVERVIEW Kevzara for subcutaneous (SC) injection is a recombinant humanized interleukin-6 (IL-6) receptor inhibitor.1 IL-6 is a pro- inflammatory cytokine that is involved in various physiologic processes. Kevzara has demonstrated efficacy and is indicated for the treatment of rheumatoid arthritis (RA) in adults with moderate to severe active RA who have had an inadequate response or intolerance to one or more disease-modifying anti-rheumatic drugs (DMARDs).1-2 Kevzara + conventional synthetic (cs)DMARD has demonstrated superior efficacy over placebo + csDMARD as assessed by American College of Rheumatology (ACR) responses, physical function, and radiographic progression. POLICY STATEMENT This policy involves the use of Kevzara. Prior authorization is recommended for pharmacy benefit coverage of Kevzara Approval is recommended for those who meet the conditions of coverage in the Criteria and Initial/Extended Approval for the diagnosis provided. Conditions Not Recommended for Approval are listed following the recommended authorization criteria. Requests for uses not listed in this policy will be reviewed for evidence of efficacy and for medical necessity on a case-by-case basis. Because of the specialized skills required for evaluation and diagnosis of patients treated with Kevzara as well as the monitoring required for adverse events and long-term efficacy, initial approval requires Kevzara be prescribed by or in consultation with a physician who specializes in the condition being treated. All approvals for initial therapy are provided for the initial approval duration noted below; if reauthorization is allowed, a response to therapy is required for continuation of therapy unless otherwise noted below. -

Study Protocol: Amendment 3
(!') GILEAU CLINICAL STUDY PROTOCOL Study Title: A Phase 3, Randomized, Double-blind, Placebo-controlled Study of Gemcitabine and Nab-paclitaxel combined with Momelotinib in Subjects with Previously Unu·eated Metastatic Pancreatic Ductal Adenocarcinoma Preceded by a Dose-fmding, Lead-in Phase Sponsor: Gilead Sciences, Inc. 333 Lakeside Drive Foster City, CA 94404 USA IND Number: 120605 EudraCT Number: 2014-004480-20 ClinicalTrials.gov Identifier: NCT021 01021 Indication: Previously unu·eated metastatic pancreatic ductal adenocarcinoma Protocol ID: GS-US-370-1296 Clinical Trials Manager: Name: PPD Telephone: PPD Gilead Medical Monitor: Name: Peter Lee, MD, PhD Telephone: PPD Clinical Program Manager: Name: PPD Telephone: PPD Protocol Version/Date: Original: 11 Febmary 2014 Amendment 1: 14 March 2014 Amendment 2: 25 August 2014 Amendment 3: 16 July 2015 CONFIDENTIALITY STATEMENT The inf01mation contained in this document, pruiicularly unpublished data, is the prope1iy or under conu·ol of Gilead Sciences, Inc., and is provided to you in confidence as an investigator, potential investigator, or consultant, for review by you, your staff, and an applicable Institutional Review Board or Independent Ethics Committee. The infonnation is only to be used by you in connection with authorized clinical studies of the investigational dmg described in the protocol. You will not disclose any of the infonnation to others without written authorization from Gilead Sciences, Inc., except to the extent necessa1y to obtain infonned consent from those persons -
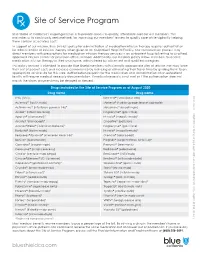
Site of Service Program
Site of Service Program Blue Shield of California’s ongoing mission is to provide access to quality, affordable care for our members. This motivates us to continually seek methods for improving our members’ access to quality care while vigilantly helping them contain associated costs. In support of our mission, Blue Shield’s policy for administration of medication infusion therapy requires authorization for administration of infusion therapy when given at an Outpatient Hospital Facility. Our authorization process may direct members with prescriptions for medication infusion therapy services in an outpatient hospital setting to qualified, approved infusion centers or physician offices instead. Additionally, our medical policy allows members to receive medication infusion therapy in their own home, administered by a licensed and qualified caregiver. This policy revision is intended to provide Blue Shield members with clinically appropriate sites of service that may lower their out-of-pocket costs and increase convenience by reducing or eliminating their travel time by guiding them to an appropriate service site for this care. Authorization requests for the medication and administration at an outpatient facility will require medical necessity documentation. If medical necessity is not met or if the authorization does not match the claim, payment may be delayed or denied. Drugs included in the Site of Service Program as of August 2020 Drug name Drug name IVIG (IVIG) Kanuma® (sebelipase alfa) Actemra® (toclizumab) Makena® (hydroxyprogesterone -

Batch 55 Block Scoping Report
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE CENTRE FOR HEALTH TECHNOLOGY EVALUATION Technology Appraisals Consultation on Batch 55 draft remits and draft scopes and summary of comments and discussions at scoping workshops Topic ID Topic title 1197 Liraglutide for preventing cardiovascular events in people with type 2 diabetes 1158 Ertugliflozin for treating type 2 diabetes 1189 Naldemedine for treating opioid-induced constipation 1194 Ixekizumab for treating active psoriatic arthritis following inadequate response to disease-modifying anti-rheumatic drugs 1198 Eltrombopag for untreated severe aplastic anaemia 1186 Mepolizumab for treating eosinophilic granulomatosis with polyangiitis 1185 Caplacizumab for treating acute acquired thrombotic thrombocytopenic purpura 1188 Erenumab for preventing migraine 1060 Tildrakizumab for treating moderate to severe plaque psoriasis TA Block scoping report – Batch 55 September 2017 Page 1 of 16 Commercial in confidence information removed © National Institute for Health and Care Excellence 2017 All rights reserved Liraglutide for preventing cardiovascular events in people with Provisional Title type 2 diabetes Topic Selection 8967 Wave / Round R227 ID Number TA ID Number 1197 Company Novo Nordisk Anticipated licensing ***CONFIDENTIAL INFORMATION REMOVED*** information To appraise the clinical and cost effectiveness of liraglutide Draft remit within its marketing authorisation for preventing cardiovascular events in people with type 2 diabetes. Following the consultation exercise, NICE is of the opinion that an appraisal of liraglutide monotherapy for preventing cardiovascular events is appropriate. A new referral is not sought because the remit for TA203 also covers this indication. Some stakeholders highlighted that the appraisal is urgent as it would give more people at high risk of CVD another therapy to reduce their risk of CVD events. -

Abstract Supplement
2018 ABSTRACT SUPPLEMENT SAN FRANCISCO June 20-23 • Marriott Marquis Federation of Clinical Immunology Societies June 20-23, 2018 San Francisco, California FOCIS 2018 Abstract Supplement TABLE OF CONTENTS Abstracts by Subject Area …………………………………………………………...……………………………….2 Allergy/asthma ……………………………………………………………………………………………………...2 Autoimmune neurologic diseases .............................................................................................................. 7 Autoimmune rheumatologic diseases ...................................................................................................... 19 Bone marrow or stem cell transplantation ............................................................................................... 37 Cytokines/chemokines ............................................................................................................................ 42 Diabetes and other autoimmune endocrine diseases .............................................................................. 47 General Autoimmunity…………………………………………………………………………………………….58 Genetics .................................................................................................................................................. 70 Immune monitoring .................................................................................................................................. 74 Immunity & infection ................................................................................................................................ 82 Immunodeficiency: