GRAS Notice 868, Coffee Fruit Extract
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Case 23 Pepsico's
BFV GROUP : Beatrice Teresa Colantoni, Francesco Morgia, Valentina Palmerio. PepsiCo’s Business Case – CASE 23 PEPSICO’S HISTORY. PepsiCo, Inc., was established in 1965 when PepsiCola and Frito-Lay shareholders agreed to a merger between the salty-snack icon and soft-drink giant. The new company was founded with annual revenues of $510 million and such well-known brands as Pepsi-Cola, Mountain Dew, Fritos, Lay’s, Cheetos, Ruffles, and Rold Gold. By 1971, PepsiCo had more than doubled its revenues to reach $1 billion. The company began to pursue growth through acquisitions outside snacks and beverages as early as 1968, but its 1977 acquisition of Pizza Hut significantly shaped the strategic direction of PepsiCo for the next 20 years. The acquisitions of Taco Bell in 1978 and Kentucky Fried Chicken in 1986 created a business portfolio described by Wayne Calloway (PepsiCo’s CEO between 1986 and 1996) as a balanced three-legged stool. Calloway believed the combination of snack foods, soft drinks, and fast food offered considerable cost sharing and skill transfer opportunities. PepsiCo strengthened its portfolio of snack foods and beverages during the 1980s and 1990s, adding also quick-service restaurant. By 1996 it had become clear to PepsiCo management that the potential strategic-fit benefits existing between restaurants and PepsiCo’s core beverage and snack businesses were difficult to capture. In 1997, CEO Roger Enrico spun off the company’s restaurants as an independent, publicly traded company to focus PepsiCo on food and beverages. Soon after the spinoff of PepsiCo’s fast-food restaurants was completed, Enrico acquired Cracker Jack, Tropicana, Smith’s Snackfood Company in Australia, SoBe teas and alternative beverages, Tasali Snack Foods (the leader in the Saudi Arabian salty-snack market), and the Quaker Oats Company. -

(Various Flavors) 20 1 2/Case Oz
Soft Drink Beverage Vending Bm ID 7081 July 1, 2017 June 30, 2019 CC No. 4600240-1730927 SUCCESSFUL BIDDER: Pepsi Beverages Company, Inc. DISTRICTWIDE - Product only (No Exclusivity Revenue and No Incentives) Product Only Pricing Bid Price Vendor Contact Information Dole Juice 100% 10 oz. plastic bottle 24/case $ 11.85 Aquafina 12 oz. plastic bottle 24/case $ 5.15 Pepsi Beverages Company Aquafina 16.9 oz. plastic bottle 24/case $ 4.64 TERMS: Net 30 Aquafina 20 oz. plastic bottle 24/case $ 8.91 VendorNo.: 130325 Gatorade 12 oz. plastic bottles 24/case $ 10.30 Orders: 1-800-963-2424 Gatorade 20 oz. plastic bottles 24/case $ 17.05 EQUIPMENT REPAIR: l-800-562-6800 Sobe Life Water Vitamiffi Enhanced Waters (Various Flavors) 20 1 2/case oz. plastic bottles $ 12.02 l BILLING QUESTIONS: 1-800-789-2626 Lipton Tea (Various Flavors) 16.9 oz. plastic bottle 24/case $ 7.21 Lipton Tea (Various Flavors) 20 oz. plastic bottle 24/case $ 12.88 Machine Refillin Inforrnation Carbonated Drinks 20 oz. plastic bottle 24/case $ 12.88 Non-Carbonated Drinks 20 oz. plastic bottle 24/case $ 12.88 Email April Stepp for refill requests. BIB Carbonated 3 gallon Per Gallon $ 8.24 BIB Non-Carbonated 3 gallon Per Gallon $ 8.24 [email protected] BIB Carbonated 5 gallon Per Gallon $ 8.24 or call CO2 Tanks Per Tank $ 28.33 Pepsi : 502-363-8884 20 oz. CSD, Lipton & Brisk $ 12.88 20 oz. Aquafina / Splash 24/Case $ 8.91 20 oz. Gatorade & G2 24/case $ 17.05 20 oz. -

Reaction of Tangerines Genotypes to Elsinoe Fawcettiiunder
Reaction of tangerines genotypes to Elsinoe fawcettii under natural infection conditions Crop Breeding and Applied Biotechnology 11: 77-81, 2011 Brazilian Society of Plant Breeding. Printed in Brazil Reaction of tangerines genotypes to Elsinoe fawcettii under natural infection conditions Marcelo Claro de Souza1*, Eduardo Sanches Stuchi2 and Antonio de Goes3 Received 11 February 2010 Accepted 30 September 2010 ABSTRACT - A citrus scab disease, caused by Elsinoe fawcettii, is currently found in all citrus areas throughout Brazil. That being, given the importance of this casual agent, the behavior of tangerines and hybrids influenced by this pathogen was evaluated under natural infection conditions. This study was performed with plants around 15 years old without irrigation; 100 fruits of three plants were collected during harvest season, using a grade scale varying from 0 (absence of symptoms) to 6 (severe symptoms) the level of disease severity was determined. Among the cultivars, citrus scab resistance was observed in Citrus deliciosa, C. tangerina, C. nobilis; a mandarin hybrid (C. nobilis x C. deliciosa) and a satsuma hybrid (C. unshiu x C. sinensis). Among the other genotypes, symptoms were observed with levels of severity ranging from 1 to 3, indicating moderate resistance. Key words: Citrus scab, citrus crop, resistant varieties. INTRODUCTION In Brazil, E. fawcettii is responsible for citrus scab. The disease is widespread in many humid, citrus-cultivating In many citrus production areas around the world, areas around the world and decreases fruit values on the Elsinoe fawcettii is one of the main fungi diseases found. fresh-fruit market (Feichtenberger et al. 1986). In young It attacks a wide variety of citrus species and cultivars, plants or under severe infection, it may cause significant resulting in scab disease on leaves, twigs, and fruits (Timmer fruit drop. -

Pepsico to Acquire Rockstar for $3.85B
PepsiCo to acquire Rockstar for $3.85B 12 March 2020 | News | By Kalyani Sharma PepsiCo has also entered into an agreement, which will provide approximately $0.7 billion of payments related to future tax benefits associated with the transaction PepsiCo announced that it has entered into an agreement to acquire Rockstar Energy Beverages, the popular energy drink maker, for $3.85 billion. PepsiCo Chairman and CEO, Ramon Laguarta said, "As we work to be more consumer-centric and capitalize on rising demand in the functional beverage space, this highly strategic acquisition will enable us to leverage PepsiCo's capabilities to both accelerate Rockstar's performance and unlock our ability to expand in the category with existing brands such as Mountain Dew. Over time, we expect to capture our fair share of this fast-growing, highly profitable category and create meaningful new partnerships in the energy space." Rockstar, founded in 2001, produces beverages that are designed for those who lead active lifestyles from athletes to rock stars. Rockstar products are available in over 30 flavors at convenience and grocery outlets in over 30 countries. PepsiCo has had a distribution agreement with Rockstar in North America since 2009. In addition to Rockstar, PepsiCo's energy portfolio includes Mountain Dew's Kickstart, GameFuel, and AMP. Russ Weiner, Rockstar's founder and creator of the world's first 16oz energy drink said, "We have had a strong partnership with PepsiCo for the last decade, and I'm happy to take that to the next level and join forces as one company. PepsiCo shares our competitive spirit and will invest in growing our brand even further. -

Caffeine, Energy Drinks, and Effects on the Body
Caffeine, Energy Drinks, and Effects on the Body Source 1: "Medicines in My Home: Caffeine and Your Body" by the Food and Drug Administration Caffeine Content in Common Drinks and Foods (University of Washington) Item Item size Caffeine (mg) Coffee 150 ml (5 oz) 60–150 Coffee, decaf 150 ml (5 oz) 2–5 Tea 150 ml (5 oz) 40–80 Hot Cocoa 150 ml (5 oz) 1–8 Chocolate Milk 225 ml 2–7 Jolt Cola 12 oz 100 Josta 12 oz 58 Mountain Dew 12 oz 55 Surge 12 oz 51 Diet Coca Cola 12 oz 45 Coca Cola 12 oz 64 Coca Cola Classic 12 oz 23 Dr. Pepper 12 oz 61 Mello Yellow 12 oz 35 Mr. Pibb 12 oz 27 Pepsi Cola 12 oz 43 7-Up 12 oz 0 COPYRIGHT © 2015 by Vantage Learning. All Rights Reserved. No part of this work may be used, accessed, reproduced or distributed in any form or by any means or stored in a database or any retrieval system, without the prior written permission of Vantage Learning. Caffeine, Energy Drinks, and Effects on the Body Mug Root Beer 12 oz 0 Sprite 12 oz 0 Ben & Jerry's No Fat Coffee 1 cup 85 Fudge Frozen Yogurt Starbucks Coffee Ice Cream 1 cup 40–60 Dannon Coffee Yogurt 8 oz 45 100 Grand Bar 1 bar (43 g) 11.2 Krackel Bar 1 bar (47 g) 8.5 Peanut Butter Cup 1 pack (51 g) 5.6 Kit Kat Bar 1 bar (46 g) 5 Raisinets 10 pieces (10 g) 2.5 Butterfinger Bar 1 bar (61 g) 2.4 Baby Ruth Bar 1 bag (60 g) 2.4 Special Dark Chocolate Bar 1 bar (41 g) 31 Chocolate Brownie 1.25 oz 8 Chocolate Chip Cookie 30 g 3–5 Chocolate Ice Cream 50 g 2–5 Milk Chocolate 1 oz 1–15 Bittersweet Chocolate 1 oz 5–35 Source 3: Excerpt from "CAERS Adverse Events Reports Allegedly Related to 5 Hour Energy" by The Food and Drug Administration http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofFoods/CFSAN/CFSANFOIAElectron icReadingRoom/UCM328270.pdf Received Symptoms Outcomes Date ANAPHYLACTIC SHOCK, LIFE THREATENING, VISITED AN ER, URTICARIA, DYSPNOEA, VISITED A HEALTH CARE PROVIDER, 3/24/2011 LETHARGY, HYPERSOMNIA, OTHER SERIOUS (IMPORTANT MEDICAL ASTHENIA EVENTS) RENAL IMPAIRMENT, FOETAL LIFE THREATENING, CONGENITAL 4/21/2011 DISTRESS SYNDROME ANOMALY COPYRIGHT © 2015 by Vantage Learning. -

Comparison of Economic Efficiency Between in Vitro and Field Methods for Vegetative Propagation of Coffea Canephora
Australian Journal of Basic and Applied Sciences, 9(20) June 2015, Pages: 1-7 ISSN:1991-8178 Australian Journal of Basic and Applied Sciences Journal home page: www.ajbasweb.com Comparison of Economic Efficiency between in Vitro and Field Methods for Vegetative Propagation of Coffea Canephora 1Mauricio Reginaldo Alves dos Santos, 2Carolina Augusto de Souza, 3Josilene Felix da Rocha, 4Leonardo Ventura de Araujo and 5Marcelo Curitiba Espindula 1,2,4,5 Embrapa Rondonia, Box.127. Porto Velho. Brazil. 76815-800 3Universidade Federal de Lavras, Box.3037. Lavras. Brazil. 37200-000 ARTICLE INFO ABSTRACT Article history: Background: Coffea canephora is a rustic species of coffee which is drought tolerant Received 16 April 2015 and resistant to diseases that commonly affect C. arabica. It contributes to about 35% of Accepted 12 June 2015 the world coffee production and is advantageous for the soluble coffee industry. Available online 1 July 2015 Propagation of C. canephora by seeds is undesirable because this method results in high heterozygosity and great genetic variability among populations. Its vegetative Keywords: propagation is an alternative to avoid this issue and has been successfully achieved by Coffee, Somatic embryogenesis, both in vitro and field methods, mainly by somatic embryogenesis and rooting of Rooting of cuttings, Production cost. cuttings, respectively. Objective: The objective of this study was to approach the viability of the two forms of propagation, comparing cost and time for the production of new plantlets and number of plantlets produced in each propagation system. Results: The final cost of a plantlet produced under in vitro conditions is US$ 0.23, while under field conditions is US$ 0.12, human resources being the highest cost in both systems. -

Summary of the Application: DRIED COFFEE CHERRY of Coffea Sp
Summary of the application: DRIED COFFEE CHERRY of Coffea sp. (Cascara) Applicant: LUIGI LAVAZZA SpA, Via Bologna,32, 10152 Torino, ITALY This is an application for notification to place DRIED COFFEE CHERRY (commonly referred to as Cascara), from Coffea sp., on the market in the European Union (EU) for use as an ingredient in a herbal tea/infusion referred to as “Coffee cherry tea”, which is prepared by brewing the DRIED COFFEE CHERRY. This application is submitted pursuant to Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, and specifically corresponds to the category covered in Article 2 (iv) “food consisting of, isolated from or produced from plants or their parts, except when the food has a history of safe food use within the Union and is consisting of, isolated from or produced from a plant or a variety of the same species obtained by traditional propagating practices which have been used for food production within the Union before 15 May 1997.” The application was prepared according to the European Food Safety Authority (EFSA) guidance on the preparation and presentation of an application for notification of a Traditional Food in the context of Regulation (EU) 2015/2283. Evidence of the consumption of “Coffee cherry tea” in Ethiopia dates back to before the 9th century; “Coffee cherry tea” is indeed commonly used as substitute for coffee in Ethiopia and Yemen. In the scientific literature and in this notification, the term DRIED COFFEE CHERRY refers to the outer part of coffee fruit, the husk and pulp, and accounts for almost 45% of the fresh berry. -

Effect of Roasting Levels and Drying Process of Coffea Canephora on the Quality of Bioactive Compounds and Cytotoxicity
International Journal of Molecular Sciences Article Effect of Roasting Levels and Drying Process of Coffea canephora on the Quality of Bioactive Compounds and Cytotoxicity Deborah Bauer 1 , Joel Abreu 1 , Nathállia Jordão 2, Jeane Santos da Rosa 3, Otniel Freitas-Silva 3 and Anderson Teodoro 1,* 1 Laboratory of Functional Foods—Universidade Federal do Estado do Rio de Janeiro, Rio de Janeiro 22290-240, Brazil; [email protected] (D.B.); [email protected] (J.A.) 2 Food Science Departament, Universidade Federal do Estado do Rio de Janeiro, Rio de Janeiro 22290-240, Brazil; [email protected] or [email protected] 3 Empresa Brasileira de Pesquisa Agropecuária—Embrapa Agroindústria de Alimentos, Rio de Janeiro 23020-470, Brazil; [email protected] or [email protected] (J.S.d.R.); [email protected] (O.F.-S.) * Correspondence: [email protected]; Tel.: +55-21-2542-7236 Received: 30 August 2018; Accepted: 17 October 2018; Published: 31 October 2018 Abstract: Coffee is a popular drink consumed all over the world. Besides its long-recognized stimulant effect, it has important nutritional and health effects. However, the type of bean processing modifies the composition of brewed coffee and possibly its bioactivity. In this study, extracts obtained from green and roasted beans of Coffea canephora (Coffea canephora var. robusta) were submitted to spray- or freeze-drying and were tested for antiproliferative activity, using MTT assay, and their influence on the cell cycle and apoptosis by flow cytometry analysis. Moreover, colors and nutrient contents were measured to identify the changes due to the roasting process. -

VU Research Portal
VU Research Portal Levels of cafestol, kahweol, and related diterpenoids in wild species of the coffee plant coffea de Roos, B.; van der Weg, G.; Urgert, R.; van de Bovenkamp, P.; Charrier, A.; Katan, M.B. published in Journal of Agricultural and Food Chemistry 1997 document version Publisher's PDF, also known as Version of record Link to publication in VU Research Portal citation for published version (APA) de Roos, B., van der Weg, G., Urgert, R., van de Bovenkamp, P., Charrier, A., & Katan, M. B. (1997). Levels of cafestol, kahweol, and related diterpenoids in wild species of the coffee plant coffea. Journal of Agricultural and Food Chemistry, 45, 3065-9. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. E-mail address: [email protected] Download date: 01. Oct. 2021 J. Agric. Food Chem. 1997, 45, 3065−3069 3065 Levels of Cafestol, Kahweol, and Related Diterpenoids in Wild Species of the Coffee Plant Coffea Baukje de Roos,† Guido van der Weg,† Rob Urgert,† Peter van de Bovenkamp,† Andre´ Charrier,‡ and Martijn B. -

Chapter 1 Definitions and Classifications for Fruit and Vegetables
Chapter 1 Definitions and classifications for fruit and vegetables In the broadest sense, the botani- Botanical and culinary cal term vegetable refers to any plant, definitions edible or not, including trees, bushes, vines and vascular plants, and Botanical definitions distinguishes plant material from ani- Broadly, the botanical term fruit refers mal material and from inorganic to the mature ovary of a plant, matter. There are two slightly different including its seeds, covering and botanical definitions for the term any closely connected tissue, without vegetable as it relates to food. any consideration of whether these According to one, a vegetable is a are edible. As related to food, the plant cultivated for its edible part(s); IT botanical term fruit refers to the edible M according to the other, a vegetable is part of a plant that consists of the the edible part(s) of a plant, such as seeds and surrounding tissues. This the stems and stalk (celery), root includes fleshy fruits (such as blue- (carrot), tuber (potato), bulb (onion), berries, cantaloupe, poach, pumpkin, leaves (spinach, lettuce), flower (globe tomato) and dry fruits, where the artichoke), fruit (apple, cucumber, ripened ovary wall becomes papery, pumpkin, strawberries, tomato) or leathery, or woody as with cereal seeds (beans, peas). The latter grains, pulses (mature beans and definition includes fruits as a subset of peas) and nuts. vegetables. Definition of fruit and vegetables applicable in epidemiological studies, Fruit and vegetables Edible plant foods excluding -
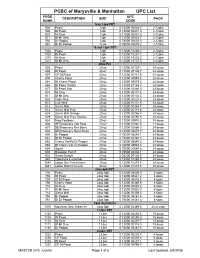
MASTER UPC June08
PCBC of Marysville & Manhattan UPC List PROD UPC DESCRIPTION SIZE PACK NUMB CODE 12oz 12pk PET 502 Pepsi 12pk 0 12000 00830 6 2-12pks 503 Dt Pepsi 12pk 0 12000 00831 3 2-12pks 510 Mt Dew 12pk 0 12000 00832 0 2-12pks 511 Dt Mt Dew 12pk 0 12000 00833 7 2-12pks 550 Dr Pepper 12pk 0 78000 00602 5 2-12pks 551 Dt Dr Pepper 12 pk 0 78000 00603 2 2-12pks 16.9oz 12pk PET 1302 Pepsi 12pk 0 12000 10200 4 2-12pks 1303 Dt Pepsi 12pk 0 12000 10201 1 2-12pks 1310 Mt Dew 12pk 0 12000 10203 5 2-12pks 1311 Dt Mt Dew 12pk 0 12000 10737 5 2-12pks 20oz Pet 602 Pepsi 20 oz 0 12000 00129 1 24 loose 603 Dt Pepsi 20 oz 0 12000 00130 7 24 loose 607 CF Dt Pepsi 20 oz 0 12000 00121 5 24 loose 690 Cherry Pepsi 20 oz 0 12000 00559 6 24 loose 691 Dt Cherry Pepsi 20 oz 0 12000 00579 4 24 loose 686 Dt Pepsi Vanilla 20 oz 0 12000 81189 0 24 loose 677 Dt Pepsi Max 20 oz 0 12000 01880 0 24 loose 610 Mt Dew 20 oz 0 12000 00131 4 24 loose 611 Dt Mt Dew 20 oz 0 12000 00134 5 24 loose 692 Code Red 20 oz 0 12000 00224 3 24 loose 618 Live Wire 20 oz 0 12000 81131 9 24 loose 613 Sierra Mist 20 oz 0 12000 00354 7 24 loose 612 Sierra Mist Free 20 oz 0 12000 20115 8 24 loose 628 Sierra Mist Orange 20 oz 0 12000 02786 4 24 loose 629 Sierra Mist Free Orange 20 oz 0 12000 02794 9 24 loose 634 Mug Rootbeer 20 oz 0 12000 00910 5 24 loose 636 DEWmocracy Volt Rasp 20 oz 0 12000 02862 5 24 loose 637 DEWmocracy Rev Berry 20 oz 0 12000 02866 3 24 loose 638 DEWmocracy Nova Straw 20 oz 0 12000 02870 0 24 loose 650 Dr Pepper 20 oz 0 78000 08240 1 24 loose 651 Dt Dr Pepper 20 oz 0 78000 08340 -

AMP Media Plan: Amplify Your Night!
AMPlify Your Night! 1 RUNNING HEAD: AMPlify Your Night! AMP Media Plan: AMPlify Your Night! Team: MESH (Mary Norton, Eric Davis, Stephen Allen, Holly Schaefer) TEL-R440 Students 2:45-5:30 p.m. Thursday Class Indiana University Southeast Department of Communication Studies New Albany, IN 47150 AMPlify Your Night! 2 Table of Contents I. Plan Summary (Page 3) II. The Problem (Page 3) III. Situation Analysis • Target Research (Page 4) • Market Information (Page 6) • Creative and Media Background (Page 9) • SWOT Analysis (Page 10) IV. The Solution • Creative Strategy (Page 11) • Media Objectives and Strategies (Page 12) AMPlify Your Night! 3 I. Plan Summary AMP is an energy drink product that has been in desperate need of a re-branding. The current brand appeals mainly toward males. This is because of the masculine look of the product, as well as its current advertising being geared towards males who play sports. Its advertisements mainly list the product as being able to enhance ones skills and endurance on the playing field. Due to this way of advertising, other demographics are left out. Our plan would shift the focus to other demographics, specifically toward women. Our solution to this would be to re-brand the product to have two separate lines: one for women and one for men. The male product line will stay the same as the current line that is available now. However the women's product line will feature a sleek new look that is both feminine and attractive. The advertising for this brand new line will gear the product towards those who enjoy the nightlife.