Factors Affecting Feeding and Brooding of Brown Thrasher Nestlings.-The Nest- Ling Period Is a Particularly Stressful Time in the Lives of Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predation by Gray Catbird on Brown Thrasher Eggs
March 2004 Notes 101 PREDATION BY GRAY CATBIRD ON BROWN THRASHER EGGS JAMES W. RIVERS* AND BRETT K. SANDERCOCK Kansas Cooperative Fish and Wildlife Research Unit, Division of Biology, Kansas State University, Manhattan, KS 66506 (JWR) Division of Biology, Kansas State University, Manhattan, KS 66506 (BKS) Present address of JWR: Department of Ecology, Evolution, and Marine Biology, University of California, Santa Barbara, CA 93106 *Correspondent: [email protected] ABSTRACT The gray catbird (Dumetella carolinensis) has been documented visiting and breaking the eggs of arti®cial nests, but the implications of such observations are unclear because there is little cost in depredating an undefended nest. During the summer of 2001 at Konza Prairie Bio- logical Station, Kansas, we videotaped a gray catbird that broke and consumed at least 1 egg in a brown thrasher (Toxostoma rufum) nest. Our observation was consistent with egg predation because the catbird consumed the contents of the damaged egg after breaking it. The large difference in body mass suggests that a catbird (37 g) destroying eggs in a thrasher (69 g) nest might risk injury if caught in the act of predation and might explain why egg predation by catbirds has been poorly documented. Our observation indicated that the catbird should be considered as an egg predator of natural nests and that single-egg predation of songbird nests should not be attributed to egg removal by female brown-headed cowbirds (Molothrus ater) without additional evidence. RESUMEN El paÂjaro gato gris (Dumetella carolinensis) ha sido documentado visitando y rompien- do los huevos de nidos arti®ciales, pero las implicaciones de dichas observaciones no son claras porque hay poco costo por depredar un nido sin defensa. -

Birds of Perry County Contact Us the Tell City Ranger District of the Hoosier National Forest Is Open 8-4:30 Monday Through Friday to Serve Visitors
Birds of Perry County Contact Us The Tell City Ranger District of the Hoosier National Forest is open 8-4:30 Monday through Friday to serve visitors. Tell City Ranger District 248 15th Street Tell City, IN 47586 812-547-7051 Federal relay system for the deaf and hearing impaired: 1-800-877-8339 website: www.fs.usda.gov/hoosier Great Bllue Heron Tufted Titmouse __________________________ vV USDA is an equal opportunity provider and employer. America’s Great Outdoors Last updated 11/2011 Forest Service United States Department of Agriculture The third and fourth columns are the genus and Using the Checklist species of the bird. The fifth column shows the The first column after the bird’s common name is bird’s status in Indiana as of 2009. (Available at http:// evidence of the bird’s breeding status in our area. www.in.gov/dnr/fishwild/files/Birds_Of_Indiana.pdf) CO = Confirmed breeding evidence FC = Federal Candidate FE = Federal Endangered PR = Probable breeding evidence FT = Federal Threatened SC - State Special Concern PO = Possible breeding evidence SE = State Endangered X = Exotic/Introduced OB = Observed, no breeding evidence Bird abundance will vary seasonally, and often from This shows highest breeding evidence value from year-to-year as well. Actual abundance is often dis- published 1985-1990 breeding bird atlas data and tinct from detectability. Some species may be com- draft 2005-2010 atlas data. (Available at http://www. mon but secretive and only rarely seen. Others may pwrc.usgs.gov/bba/) be numerically sparse, yet highly -

Aullwood's Birds (PDF)
Aullwood's Bird List This list was collected over many years and includes birds that have been seen at or very near Aullwood. The list includes some which are seen only every other year or so, along with others that are seen year around. Ciconiiformes Great blue heron Green heron Black-crowned night heron Anseriformes Canada goose Mallard Blue-winged teal Wood duck Falconiformes Turkey vulture Osprey Sharp-shinned hawk Cooper's hawk Red-tailed hawk Red-shouldered hawk Broad-winged hawk Rough-legged hawk Marsh hawk American kestrel Galliformes Bobwhite Ring-necked pheasant Gruiformes Sandhill crane American coot Charadriformes Killdeer American woodcock Common snipe Spotted sandpiper Solitary sandpiper Ring-billed gull Columbiformes Rock dove Mourning dove Cuculiformes Yellow-billed cuckoo Strigiformes Screech owl Great horned owl Barred owl Saw-whet owl Caprimulgiformes Common nighthawk Apodiformes Chimney swift Ruby-throated hummingbird Coraciformes Belted kinghisher Piciformes Common flicker Pileated woodpecker Red-bellied woodpecker Red-headed woodpecker Yellow-bellied sapsucker Hairy woodpecker Downy woodpecker Passeriformes Eastern kingbird Great crested flycatcher Eastern phoebe Yellow-bellied flycatcher Acadian flycatcher Willow flycatcher Least flycatcher Eastern wood pewee Olive-sided flycatcher Tree swallow Bank swallow Rough-winged swallow Barn swallow Purple martin Blue jay Common crow Black-capped chickadee Carolina chickadee Tufted titmouse White-breasted nuthatch Red-breasted nuthatch Brown creeper House wren Winter wren -

Trematode Parasites of the Brown Thrasher, Toxostoma Rufum, from Dickinson County, Lowa
Proceedings of the Iowa Academy of Science Volume 77 Annual Issue Article 29 1970 Trematode Parasites of the Brown Thrasher, Toxostoma rufum, from Dickinson County, lowa Susan Peet Iowa State University Martin J. Ulmer Iowa State University Let us know how access to this document benefits ouy Copyright ©1970 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Peet, Susan and Ulmer, Martin J. (1970) "Trematode Parasites of the Brown Thrasher, Toxostoma rufum, from Dickinson County, lowa," Proceedings of the Iowa Academy of Science, 77(1), 196-199. Available at: https://scholarworks.uni.edu/pias/vol77/iss1/29 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Peet and Ulmer: Trematode Parasites of the Brown Thrasher, Toxostoma rufum, from Trematode Parasites of the Brown Thrasher, Toxostoma rufum, from Dickinson County, lowa1 SusAN PEET and MARTIN J. ULMER" Abstract. Nineteen Brown Thrashers (seven adults, 12 immatures) were carefully examined for trematodes during the summer of 1968. Tre matodes were recovered from ten hosts and include the following: Bra chylaima sp. (intestine) ; Brachylecith um exochocotyle (gall bladder) ; Lutztrema microstomum (gall bladder); Lyperosomum oswaldoi (gall blad der and bile ducts) ; Tanaisia zarudnyi (ureter) ; U rogonimus certhiae (cloaca); and U. dryobatae (cloaca). All trematodes represent new locality records and all but B. exochocotyle and L. -

The Journal of Louisiana Ornithology
The Journal of Louisiana Ornithology VOLUME 9 NOVEMBER 2012 PUBLISHED BY THE LOUISIANA ORNITHOLOGICAL SOCIETY The Journal of Louisiana Ornithology is published on-line annually by the Louisiana Ornithological Society. Address business correspondence to: Secretary-Treasurer Judith O’Neale, 504 Whitebark Lane, Lafayette, LA 70508. The Editor is Jennifer O. Coulson. Manuscripts are solicited on any aspect of Louisiana ornithology, or on any issue which is pertinent to an understanding of the birdlife of Louisiana. Articles, news, and notes from the field, and literature reviews may address questions of identification, seasonal, or geographic distribution, behavior, ecology, conservation, or related matters. Articles submitted are expected to be the original work of the authors and shall not have been published elsewhere. Manuscripts containing research results will be reviewed by one or more referees. Manuscripts must be typed and formatted using an 8.5" x 11" page size, with 1" margins and double-spaced throughout, including literature citations, tables, and captions to figures. All photographs, graphics, line drawings, or half-tones must be provided in high quality digital form. Send photographs and artwork as separate files (.jpeg, .tiff, and .pdf are acceptable file types). Do not embed these files in the manuscript document. Full color photographs and artwork are welcomed and may be suggested as cover art. Submit manuscripts and accompanying files as email attachments or on computer discs in Microsoft Word. Inquiries should be directed to the editor. Authors should adhere to the style of The Auk. Scientific and common names must follow the A.O.U. Checklist and its supplements. Manuscripts should be sent to the Editor: Jennifer O. -
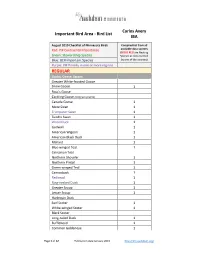
Bird List IBA
Carlos Avery Important Bird Area - Bird List IBA August 2010 Checklist of Minnesota Birds Compiled list from all Red: PIF Continental Importance available data sources (BOLD RED are Nesting Green: Stewardship Species Species as documented Blue: BCR Important Species by one of the sources) Purple: PIF Priority in one or more regions REGULAR Ducks, Geese, Swans Greater White-fronted Goose Snow Goose 1 Ross's Goose Cackling Goose (tallgrass prairie) Canada Goose 1 Mute Swan 1 Trumpeter Swan 1 Tundra Swan 1 Wood Duck 1 Gadwall 1 American Wigeon 1 American Black Duck 1 Mallard 1 Blue-winged Teal 1 Cinnamon Teal Northern Shoveler 1 Northern Pintail 1 Green-winged Teal 1 Canvasback 1 Redhead 1 Ring-necked Duck 1 Greater Scaup 1 Lesser Scaup 1 Harlequin Duck Surf Scoter 1 White-winged Scoter 1 Black Scoter Long-tailed Duck 1 Bufflehead 1 Common Goldeneye 1 Page 1 of 12 Publication date January 2015 http://mn.audubon.org/ Carlos Avery Important Bird Area - Bird List IBA August 2010 Checklist of Minnesota Birds Compiled list from all Red: PIF Continental Importance available data sources (BOLD RED are Nesting Green: Stewardship Species Species as documented Blue: BCR Important Species by one of the sources) Purple: PIF Priority in one or more regions Hooded Merganser 1 Common Merganser 1 Red-breasted Merganser 1 Ruddy Duck 1 Partridge, Grouse, Turkey Gray Partridge 1 Ring-necked Pheasant 1 Ruffed Grouse 1 Spruce Grouse Sharp-tailed Grouse Greater Prairie-Chicken Wild Turkey 1 Loons Red-throated Loon Pacific Loon Common Loon 1 Grebes Pied-billed -

Brown Thrasher Toxostoma Rufum
Brown Thrasher Toxostoma rufum Brown Thrashers occupy shrubby edge and successional Some thrasher nests are placed on the ground under dense habitats, frequently sharing their breeding territories with Gray cover such as thick bushes and the branches of fallen trees. Catbirds. Nesting thrashers prefer fallow fields dominated by However, most nests are placed at heights of 2–10 feet in dense dense shrubby thickets, the brushy margins of woodlands, and woody cover, particularly hawthorn trees, tangles composed of shrubby thickets along fencerows and roadsides. Multiflora rose roses and other thorny shrubs, and grape vines (Trautman 1940, hedges bordering farm fields provide acceptable habitats within Williams 1950). intensively farmed areas where other brushy nesting sites are scarce. However, thrashers do not exhibit the same adaptability in their choice of breeding habitats as is displayed by catbirds. Thrashers definitely prefer dry upland sites and only rarely occur near water. They avoid urban residential areas, although thrashers will occupy brushy thickets bordering rural residences. This species also normally avoids woodland openings and other wooded habitats, but a few pairs may be found in formerly grazed woodlots with dense shrubby cover. Despite their more restrictive habitat requirements, breeding Brown Thrashers are widely distributed summer residents in Ohio. The Atlas Project produced records from 748 priority blocks representing 98% of the statewide total. They were fairly evenly distributed within every physiographic region with records from every priority block in the Illinoian Till Plain region and 94.7–98.6% of the blocks in the other regions. Their relative abundance on Ohio Breeding Bird Surveys exhibits a slightly different pattern. -

Habitat Use and Nesting of Breeding Brown Thrashers Joshua Castle, Eastern Kentucky University Mentors: Drs
Habitat Use and Nesting of Breeding Brown Thrashers Joshua Castle, Eastern Kentucky University Mentors: Drs. David Brown and Kelly Watson. Coauthor: Kasie Bradley condition (Gleditsch & Carlo 2014). Further research is needed into the mechanisms Introduction leading to higher predation rates and the Across North America, the impacts these invasive shrubs have on avian populations of bird species are in decline. communities, leaving the classification as an Results from long-term surveys revealed a ecological trap a controversial subject. net loss of approximately 3 billion birds One Kentucky species that utilizes across all biomes, a decline of 29% since shrubs in its breeding season is the Brown 1970 (Rosenberg & et al. 2019). This Thrasher (Toxostoma rufum). The North substantial population decline is not American Breeding Bird Survey reported a restricted to threatened and endangered 41% decline in the population of Brown species but includes many common birds Thrashers from 1966 to 2015 (Cavitt & Haas that may impact environmental function, 2014). Along with habitat invasion by exotic which could lead to devastating plants, this and other species also face a consequences. If we continue to ignore the decline in breeding habitat. This decrease in decline of common bird species, North suitable breeding ground is only America will soon suffer an immense exacerbating the already steady fall of bird financial cost as more birds are added to the populations across the continent. endangered species list (Rosenberg & et al. 2019). This added cost will make it more We chose this species for study challenging to protect already endangered because of their early arrival in Kentucky species, and many could potentially go and their tendency to produce 2 broods extinct. -

2Nd Owl Symposium Importance of Prairie
2nd Owl Symposium Importance of Prairie Wetlands and Avian Prey to Breeding Great Horned Owls (Bubo virginianus) in Northwestern North Dakota Robert K. Murphy 1 Abstract.—Prey use by Great Horned Owls (Bubo virginianus) is documented widely in North America, but not in the vast northern Great Plains. During spring through early summer 1986-1987, I recorded 2,900 prey items at 22 Great Horned Owl nesting areas in the prairie pothole farm- and rangelands of northwestern North Dakota. The owls relied heavily on wetland-dependent prey species (overall, 57 percent by number and 76 percent biomass) especially ducks (Anserinae) and rails (Rallidae). Far more avian (65 percent by number and 84 percent biomass) and less mammalian prey were used than typically reported. Variation in diet composition among owl families was not explained well by nesting area habitat, and was dominated by prey from wetlands regardless of wetland habitat availability. Diets of Great Horned Owls (Bubo virginianus) (Murphy 1993, Sargeant et al. 1993). The are better documented than those of most increase in this generalist predator may have other North American strigiforms. The owl implications for population dynamics of species preys mainly on small to mid-size mammals on which it preys. My objectives were to quan- especially small rodents and leporids tify diet composition of breeding Great Horned (Errington et al. 1940; Korschgen and Stuart Owls in an area of mixed farm- and rangeland 1972; McInvaille and Keith 1974; Marti and in the northern Great Plains, to assess varia- Kochert 1995, 1996; Voous 1988) although its tion in prey use among owl pairs, and to test list of prey includes diverse sizes and taxa (see whether such variation is explained by habitat Bent 1938). -

Gray Catbird, Northern Mockingbird and Brown Thrasher
Wildlife Note — 51 LDR0103 Gray Catbird, Northern Mockingbird and Brown Thrasher by Chuck Fergus Gray Catbird These three species are among the most vocal of our birds. All belong to Family Mimidae, the “mimic thrushes,” or “mimids,” and they often imitate the calls of other spe- cies, stringing these remembered vocalizations into long, variable songs. Family Mimidae has more than 30 spe- cies, which are found only in the New World, with most inhabiting the tropics. The mimids have long tails and short, rounded wings. The three species in the Northeast are solitary (living singly, in pairs and in family groups rather than in flocks), feed mainly on the ground and in shrubs, and generally eat insects in summer and fruits in winter. The sexes look alike. Adults are preyed upon by owls, hawks, foxes and house cats, and their nests may be raided by snakes, blue jays, crows, grackles, raccoons, opossums and squirrels. Gray Catbird (Dumetella carolinensis) — The gray cat- bird is eight to nine inches long, smaller and more slen- der than a robin, an overall dark gray with a black cap Beetles, ants, caterpillars, and chestnut around the vent. Individuals often jerk their grasshoppers, crickets and other insects tails — up, down, and in circles. The species is named are common foodstuffs. Catbirds often forage on the for its mewling call, although catbirds also deliver other ground, using their bills to flick aside leaves and twigs sounds. They migrate between breeding grounds in the while searching for insects. eastern two-thirds of North America and wintering ar- Although not as talkative as the northern mocking- eas in the coastal Southeast and Central America. -

BIRD POPULATIOR in VARIOUS ECOLOGICAL CO'n!UNITIES. C L ~ S S Frog June 29 -~Uly'6, 1946
BIRD POPULATIOR IN VARIOUS ECOLOGICAL CO'n!UNITIES. - -- - Agrie s Kugel. P' Report on i+-g c&ts of the Adv~ncedOrnitilology cl~ssfrog June 29 -~uly'6, 1946. (zoology 377-4. Cnarles Kencieign) Bird Po?-tilation In Various Bc~logicalCoarnunities. Introduction. I - The Ornithology Class of The Biological Station ,Cneboygan !tichiean, gde-rr-the _dii.._e_cCtion of S. C.Kendoigh -- visited- . - --.a represent-- - -- - ative group of six plant communities durinq the sumer of 1946, These tri2s were msde between June 29 and July 6th to determine the bird ?opulation of a given area during the nesting sehson arid to note so far as possible the raasons for their presence. Methods. The clzss working in fwo groups cruised througn the selected i area hbou@feet apbrt and recorded all birds seen an6 heard in a measurec period of time. The average distarice trht-eled was 1 nile per hour. The popul~tionwas determined by converting tne actual co-mt taken into the nsmber tnat voula be seen in ten nours in the zhme are&. Cinze tnie we t.ile nesting Eekson it vrhs assume+bt each mde seer. also meant tne pesence of a fe3de &rid thqs tile fiwl figures as given on tiie hccornpanyi;-g clikrt ceriote the potential number of pair: of birds for ezca conrn-ai'y. A similar co-at -$;as worked out by Cr, Eendeigh for a linear disthnce of 12 ~ilesrhtner tmn trle nurnber for l"ilo;;rs, Grasslbnd. Arch This grzssy field on tne I~llztonroid boarcieree on one side by the road an6 on t'm otner by & young Aspen gro:vtn nis h speciec list of 6 gr&:slbnd bird5 anu 7 low ~kr~bbirds of tne for.est edse, The herbiceous zlarits include Asclepi~ssyrizca, Potentilla recth, The low silrubs ~omewntit ~~htterednetr tine young Aspens were pornus Ztolonifera, Hubus allegneniensis and Edix species. -

Learn About Texas Birds Activity Book
Learn about . A Learning and Activity Book Color your own guide to the birds that wing their way across the plains, hills, forests, deserts and mountains of Texas. Text Mark W. Lockwood Conservation Biologist, Natural Resource Program Editorial Direction Georg Zappler Art Director Elena T. Ivy Educational Consultants Juliann Pool Beverly Morrell © 1997 Texas Parks and Wildlife 4200 Smith School Road Austin, Texas 78744 PWD BK P4000-038 10/97 All rights reserved. No part of this work covered by the copyright hereon may be reproduced or used in any form or by any means – graphic, electronic, or mechanical, including photocopying, recording, taping, or information storage and retrieval systems – without written permission of the publisher. Another "Learn about Texas" publication from TEXAS PARKS AND WILDLIFE PRESS ISBN- 1-885696-17-5 Key to the Cover 4 8 1 2 5 9 3 6 7 14 16 10 13 20 19 15 11 12 17 18 19 21 24 23 20 22 26 28 31 25 29 27 30 ©TPWPress 1997 1 Great Kiskadee 16 Blue Jay 2 Carolina Wren 17 Pyrrhuloxia 3 Carolina Chickadee 18 Pyrrhuloxia 4 Altamira Oriole 19 Northern Cardinal 5 Black-capped Vireo 20 Ovenbird 6 Black-capped Vireo 21 Brown Thrasher 7Tufted Titmouse 22 Belted Kingfisher 8 Painted Bunting 23 Belted Kingfisher 9 Indigo Bunting 24 Scissor-tailed Flycatcher 10 Green Jay 25 Wood Thrush 11 Green Kingfisher 26 Ruddy Turnstone 12 Green Kingfisher 27 Long-billed Thrasher 13 Vermillion Flycatcher 28 Killdeer 14 Vermillion Flycatcher 29 Olive Sparrow 15 Blue Jay 30 Olive Sparrow 31 Great Horned Owl =female =male Texas Birds More kinds of birds have been found in Texas than any other state in the United States: just over 600 species.