Annual Review 2007
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Governance and Remuneration 2014
Governance & remuneration reportStrategic In this section Our Board 72 Our Corporate Executive Team 76 Chairman’s letter 78 Corporate governance framework 79 Board report to shareholders Oversight and stewardship in 2014 and future actions 80 remuneration & Governance Leadership and effectiveness 82 Committee reports Audit & Risk 86 Nominations 92 Corporate Responsibility 94 Remuneration report Chairman’s annual statement 96 Annual report on remuneration 97 2014 Remuneration policy report 119 Financial statements Financial Investor information Investor GSK Annual Report 2014 71 Our Board Strategic reportStrategic Diversity Experience International experience Composition Tenure (Non-Executives) % % % % Scientific 19 Global 75 Executive 19 Up to 3 years 39 % % % % Finance 31 USA 100 Non-Executive 81 3-6 years 15 % % % % Industry 50 Europe 94 Male 69 7-9 years 23 % % % EMAP 63 Female 31 Over 9 years 23 Sir Christopher Gent 66 Skills and experience Chairman Sir Christopher has many years of experience of leading global businesses and a track record of delivering outstanding performance Governance & remuneration & Governance Nationality in highly competitive industries. He was appointed Managing Director British of Vodafone plc in 1985 and then became its Chief Executive Officer Appointment date in 1997 until his retirement in 2003. Sir Christopher was also a 1 June 2004 and as Chairman Non-Executive Director of Ferrari SpA and a member of the British on 1 January 2005 Airways International Business Advisory Board. Committee membership External appointments Corporate Responsibility Sir Christopher is a Senior Adviser at Bain & Co. Committee Chairman, Nominations, Remuneration and Finance Sir Philip Hampton 61 Skills and experience Chairman Designate Prior to joining GSK, Sir Philip chaired major FTSE 100 companies including J Sainsbury plc. -

Glaxosmithkline Bangladesh Limited (GSK)
GlaxoSmithKline Bangladesh Limited (GSK) Recruitment and Selection Process of GlaxoSmithKline Bangladesh Limited: An Evaluation nd Date of Submission: 2 September, 2014 ©Daffodil International University DAFFODIL INTERNATIONAL UNIVERSITY Internship Report On Recruitment and Selection Process of GlaxoSmithKline Bangladesh Limited: An Evaluation Submitted To Dr. Zakir Hossain Dean& Professor Faculty of Business & Economics Daffodil International University Submitted By Ishrat Jahan ID: 131-14-1019 Masters of Business Administration Daffodil International University Date of Submission: 2nd September, 2014 ©Daffodil International University Letter of Transmittal September 2, 2014 To Dr. Zakir Hossain Dean & Professor Faculty of Business and Economics Daffodil International University Dhaka-1205 Subject: Submission of internship report on recruitment and selection process: An evaluation of GlaxoSmithKline Bangladesh Limited Sir, I am highly satisfied to submit my report on recruitment and selection process: an evaluation of GSK. For preparing this report I tried my best to accumulate relevant and upgraded information from available sources. In preparing this report, I tried my level best to make it a complete one and sincerely look forward to any possible correction. I am very glad because you also given the opportunity to prepare this report .I hope that this report will meet the standards of your judgments. Your Sincerely ---------------- Ishrat Jahan ©Daffodil International University i Certificate of the Supervisor This is to certify that the internship report titled ―Recruitment and Selection Process of GlaxoSmithKline Bangladesh Limited: An Evaluation‖, has been prepared by Ms. Ishrat Jahan bearing ID: 131-14-1019 under my supervision, a practical study on GlaxoSmithKline Bangladesh Limited. I think on the basic of declaration Ms. -

Annual Report 2013
Annual Report 2013 “ Being active and having a positive outlook on life is what keeps me going every day.” Overview of 2013 “ Our performance in 2013 was defined by remarkable &R D output and further delivery of sustained financial performance for our shareholders.” Please go to page 4 for more More at gsk.com Performance highlights £26.5bn £8.0bn £7.0bn £5.2bn Group turnover Core* operating profit Total operating profit Returned to shareholders 6 112.2p 112.5p 13% Major medicines approved Core* earnings per share Total earnings per share Estimated return on R&D investment 10 6 1st 1st Potential phase III study starts in 2014/15 Potential medicines with phase III data in Access to Medicines Index Pharmaceutical company to sign AllTrials expected 2014/15 campaign for research transparency Front cover story Betty, aged 65, (pictured) has Chronic “ Health is important to me, Obstructive Pulmonary Disease (COPD). She only has 25% lung capacity. This means I try to take care of my she finds even everyday tasks difficult, but medicines and inhaled oxygen allow her to health with all the tools live as normal a life as she can. Betty’s mindset I have and do the best is to stay busy and active, so every week she goes to rehab exercise classes. that I can with it.” COPD is a disease of the lungs that leads to Betty, COPD patient, damaged airways, causing them to become North Carolina, USA narrower and making it harder for air to get in and out. 210 million people around the world are estimated to have COPD. -

Gilead and Glaxo's HIV Battle Intensifies
February 14, 2017 Gilead and Glaxo’s HIV battle intensifies Madeleine Armstrong The first phase II data with Gilead’s HIV integrase inhibitor bictegravir have set up a showdown with Glaxosmithkline/Viiv Healthcare’s marketed product dolutegravir. But Glaxo is already one step ahead with data from a two-drug combo that could reduce the side-effect burden for HIV patients. Glaxo believes that this doublet could “reshape the whole game”, the group's chief executive, Andrew Witty, said on its fourth-quarter earnings call. HIV has been one of the group’s main drivers in recent quarters as it has taken market share from Gilead, which is becoming increasingly reliant on HIV as its hepatitis C franchise slows (see tables below). Bictegravir vs dolutegravir The phase II study, presented today at the Conference on Retroviruses and Opportunistic Infections in Seattle, compared bictegravir plus emtricitabine and tenofovir alafenamide – known as FTC/TAF – with dolutegravir plus FTC/TAF in 98 treatment-naive, HIV-infected adults. Both are the latest iterations of the integrase inhibitor class, and unlike older products with the same mechanism do not require boosting – taking another drug to raise circulating levels – so have a lower risk of adverse events and drug-drug interactions. The phase II trial found similar response rates with a bictegravir-containing regimen versus a dolutegravir- containing therapy, which should boost confidence in Gilead’s compound ahead of phase III readouts expected mid-year. It found a 97% response in the bictegravir arm at 24 and 48 weeks, versus 94% at week 24 and 91% at week 48 in the dolutegravir arm, but the difference was not statistically significant. -

Pharma Pricing, Non-Profit Ties Get Increasing Scrutiny from Prosecutors
REGULATORY UPDATE REGULATORY UPDATE CONSUMER DRUGS Pediatric Rare Disease Voucher Japan Wants EMA To Stay In Digital Marketing: Health Care Brands’ Program Faces Expiration, p. 12 UK Post-Brexit, p. 15 Window Into Consumers’ Lives, p. 20 Pharma intelligence Pinkpink.pharmamedtechbi.comSheetVol. 78 / No. 38 September 19, 2016 informa Mylan NV was subpoenaed for material Pharma Pricing, Non-Profit Ties Get about the pricing of its generic doxycycline and communications with competitors. And Valeant Pharmaceuticals International Inc. is Increasing Scrutiny From Prosecutors facing several probes about its pricing and BRENDA SANDBURG [email protected] patient assistance programs (see chart, p. 5). Mylan’s doxycycline price increases were called out by Sen. Bernie Sanders, I-Vt., and Rep. Elijah Cummings, D-Md., in October 2014 when they sent letters to 14 generic drug makers about the pricing of their prod- ucts. They noted that from October 2013 to April 2014, the average price charged for a 500-count bottle of 100 mg tablets had risen from $20 to $1,829, an 8,281% increase. Mylan is now under fire for repeatedly rais- ing the price of its severe allergy treatment EpiPen (epinephrine), which has increased from about $100 for a two-pack in 2008 to more than $600. Members of Congress sent a flurry of letters to the company requesting an explanation for the price hikes. And on Sept. 6, New York Attorney Gen- Shutterstock: blvdone Shutterstock: eral Eric Schneiderman announced that his office has begun an investigation into rug makers have been unable to programs, contractual agreements with Mylan with regard to EpiPen, saying a pre- shake free of government inves- pharmacy benefit managers, support of liminary review revealed that Mylan may Dtigations of their marketing and non-profit organizations, and calculation have inserted potentially anticompetitive sales practices. -

Annual Review 2005
GS2184_Review_A\W2.qxd 7/3/06 4:58 pm Page fc1 Annual Review 2005 human being Do more, feel better, live longer GS2184_Review_A\W2.qxd 9/3/06 1:28 pm Page ifc2 01 An interview with Sir Christopher Gent, Chairman, and JP Garnier, Chief Executive Officer 05 Tachi Yamada, Chairman, Research & Development, Pharmaceuticals 06 Jean Stéphenne, President and General Manager, GSK Biologicals 08 John Clarke, President, Consumer Healthcare 11 David Stout, President, Pharmaceutical Operations 14 Performance highlights 15 Business operating review 18 The Board 19 The Corporate Executive Team 20 Summary remuneration report 23 Corporate governance 24 Responsibility statements 25 Summary financial statements 26 Summary information under US GAAP 27 Shareholder information 29 Chairman and CEO’s closing letter JP Garnier (left) and Sir Christopher Gent (right) GS2184_Review_A\W2.qxd 7/3/06 5:01 pm Page 01 “Discovering important medicines, eradicating diseases, improving the quality of people’s lives and making medicines available to a greater number of people. This is what we do – and what we do matters to people.” JP Garnier, Chief Executive Officer An interview with Sir Christopher Gent, Chairman and JP Garnier, Chief Executive Officer 2005: a year of success and progress “Thanks to the efforts of our employees around the company’s pipeline is one of the largest and most world, 2005 was a very successful year for GSK,” says promising in the industry, with 149 projects in clinical JP Garnier, Chief Executive Officer. “Not only was it development (as at the end of February 2006), our best year ever from a financial standpoint, we also including 95 new chemical entities (NCEs), 29 product made substantial progress with our pipeline of line extensions (PLEs) and 25 vaccines. -

General Assembly Distr
UNITED NATIONS A General Assembly Distr. GENERAL A/HRC/11/12/Add.2 5 May 2009 Original: ENGLISH HUMAN RIGHTS COUNCIL Eleventh session Agenda item 3 PROMOTION AND PROTECTION OF ALL HUMAN RIGHTS, CIVIL, POLITICAL, ECONOMIC, SOCIAL AND CULTURAL RIGHTS, INCLUDING THE RIGHT TO DEVELOPMENT Report of the Special Rapporteur on the right of everyone to the enjoyment of the highest attainable standard of health, Paul Hunt* Annex MISSION TO GLAXOSMITHKLINE** * The present report was submitted late in order to incorporate the most recent information. Paul Hunt ended his mandate as Special Rapporteur on 31 July 2008. His report is circulated as an addendum to the report of his successor, Anand Grover, who took up the mandate on 1 August 2008. ** The summary of the present report is circulated in all official languages. The report itself, annexed to the summary, is circulated as received, in the language of submission only. GE.09-13131 (E) 130509 A/HRC/11/12/Add.2 page 2 Summary The Special Rapporteur on the right of everyone to the enjoyment of the highest attainable standard of physical and mental health visited the headquarters of GlaxoSmithKline, one of the world’s leading research-based pharmaceutical companies, in June 2008 for substantive interviews with the company’s senior management. In the Special Rapporteur’s previous reports, he examined States’ responsibilities in relation to access to medicines. However, enhancing access to medicines is a shared responsibility. The Millennium Development Goals recognize that pharmaceutical companies have a responsibility to improve access to medicines. In the present report, the Special Rapporteur outlines the responsibilities of pharmaceutical companies, including innovator, generic and biotechnology companies, with regard to the right to health in relation to access to medicines. -

2005 GSK Corporate Responsibility Report
2005 GSK Corporate Responsibility Report Introduction 1 Introduction Page Our contribution to society 1 About this report 1 CEO and Chairman’s letter 3 Managing corporate responsibility 4 Why CR is important to GSK 4 CR governance 4 Our CR principles 6 Stakeholder engagement 7 Stakeholder feedback 7 How we are responding 9 Government and external affairs 10 Membership of trade associations 10 US lobbying expenditures 10 Political donations 10 Our position on key issues 11 Patient advocacy 11 2 Access to medicine 3 Research 4 Ethical conduct 5 Employment practices 6 Human rights 7 Environment 8 Community investment 9 Data summary 1 1 GSK CORPORATE RESPONSIBILITY REPORT 2005 Introduction Welcome to GSK’s Corporate Responsibility Report 2005. This report explains our approach to the wide range of social, ethical and environmental issues associated with our business and reports our performance in 2005. The full web-based report is available on www.gsk.com GSK is a research-based pharmaceutical company, with operations in 119 countries. We make prescription medicines, vaccines, over-the-counter medicines, and consumer healthcare products. Our business accounts for 6.3% of the world’s pharmaceutical market. We have strong positions in several therapeutic areas including anti-infectives, asthma, cancer, cardiovascular, depression, diabetes, HIV/AIDS and urology. For an overview of our business, see our Annual Report. OUR CONTRIBUTION TO SOCIETY This report explains our approach to the significant In the last 80 years, medicines and vaccines have corporate responsibility issues for our business, including: transformed millions of lives. They have helped to increase • Access to medicines – how we make our medicines life expectancy and lowered death rates from conditions accessible to poor patients in developed and developing such as heart disease, stroke and cancer. -

In This Section
Strategic report In this section Chairman’s statement 2 CEO’s review 4 Business overview 6 The global context 8 Our business model 12 Our strategic priorities 14 How we performed 16 Risk management 18 Grow 20 Deliver 32 Simplify 44 Our financial architecture 48 Responsible business 50 Financial review 58 Strategic report Chairman’s statement Chairman’s statement To shareholders The value of the significant changes that have been made in recent years is evidenced in our performance this year “ Since Sir Andrew became It is clear from the following pages that Through the Audit & Risk Committee, we the Group made good progress against oversee the issues and challenges faced by CEO, the company has its strategy in 2013. management, and encourage the creation of an environment in which GSK can achieve The Board believes the business is seeing returned £30 billion its strategic ambitions in a responsible and the benefits of the significant changes the sustainable manner. to shareholders.” management team has driven over recent years to deliver sustainable growth, reduce risk and I have no doubt that commercial success is enhance returns to shareholders. directly linked to operating in a responsible way and which meets the changing expectations of The notably strong performance from the society. In this respect, the company continues R&D organisation in 2013 – with six major to adopt industry-leading positions on a range new product approvals in areas including of issues. respiratory disease, HIV and cancer – is critical to the longer-term prospects of the The announcement of plans during 2013 to Group. -
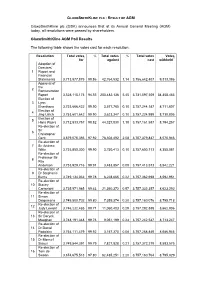
Voting/Poll Results
GLAXOSMITHKLINE PLC - RESULT OF AGM GlaxoSmithKline plc (GSK) announces that at its Annual General Meeting (AGM) today, all resolutions were passed by shareholders. GlaxoSmithKline AGM Poll Results The following table shows the votes cast for each resolution: Resolution Total votes % Total votes % Total votes Votes for* against cast withheld** Adoption of Directors’ 1 Report and Financial Statements 3,713,877,875 98.86 42,764,532 1.14 3,756,642,407 9,313,386 Approval of the 2 Remuneration Report 3,528,115,173 94.55 203,482,136 5.45 3,731,597,309 34,358,483 Election of 3 Lynn Elsenhans 3,753,666,422 99.90 3,577,765 0.10 3,757,244,187 8,711,607 Election of 4 Jing Ulrich 3,753,601,642 99.90 3,623,347 0.10 3,757,224,989 8,730,805 Election of 5 Hans Wijers 3,712,833,757 98.82 44,327,830 1.18 3,757,161,587 8,794,257 Re-election of Sir 6 Christopher Gent 3,679,075,355 97.92 78,304,492 2.08 3,757,379,847 8,575,946 Re-election of 7 Sir Andrew Witty 3,753,850,300 99.90 3,750,413 0.10 3,757,600,713 8,355,081 Re-election of Professor Sir 8 Roy Anderson 3,753,929,716 99.91 3,483,857 0.09 3,757,413,573 8,542,221 Re-election of 9 Dr Stephanie Burns 3,749,134,033 99.78 8,228,665 0.22 3,757,362,698 8,592,951 Re-election of 10 Stacey Cartwright 3,735,971,985 99.43 21,360,372 0.57 3,757,332,357 8,623,292 Re-election of 11 Simon Dingemans 3,749,800,702 99.80 7,359,374 0.20 3,757,160,076 8,795,718 Re-election of 12 Judy Lewent 3,746,232,485 99.71 11,060,403 0.29 3,757,292,888 8,662,906 Re-election of 13 Sir Deryck Maughan 3,748,191,348 99.76 9,051,199 0.24 -

Glaxosmithkline Plc Annual Report for the Year Ended 31St December 2000
GlaxoSmithKline 01 GlaxoSmithKline plc Annual Report for the year ended 31st December 2000 Contents Report of the Directors 02 Financial summary 03 Joint statement by the Chairman and the Chief Executive Officer 05 Description of business 29 Corporate governance 37 Remuneration report 47 Operating and financial review and prospects 69 Financial statements 70 Directors’ statements of responsibility 71 Report by the auditors 72 Consolidated statement of profit and loss 72 Consolidated statement of total recognised gains and losses 74 Consolidated statement of cash flow 76 Consolidated balance sheet 76 Reconciliation of movements in equity shareholders’ funds 77 Company balance sheet 78 Notes to the financial statements 136 Group companies 142 Principal financial statements in US$ 144 Financial record 153 Investor information 154 Shareholder return 156 Taxation information for shareholders 157 Shareholder information 158 Share capital 160 Cross reference to Form 20-F 162 Glossary of terms The Annual Report was approved by the Board 163 Index of Directors on 22nd March 2001 and published on 12th April 2001. Contact details 02 GlaxoSmithKline Financial summary 2000 1999 Increase Business performance £m £m CER % £ % Sales 18,079 16,164 9 12 Trading profit 5,026 4,378 12 15 Profit before taxation 5,327 4,708 11 13 Earnings/Net income 3,697 3,222 13 15 Earnings per Ordinary Share 61.0p 52.7p 14 16 Total results Profit before taxation 6,029 4,236 Earnings/Net income 4,154 2,859 Earnings per Ordinary Share 68.5p 46.7p Business performance: results exclude merger items and restructuring costs; 1999 sales and trading profit exclude the Healthcare Services businesses which were disposed of in 1999. -

Corporate Responsibility Report 2008
Corporate Responsibility Report 2008 Strength through responsibility Summary Reed Elsevier Corporate Responsibility Report 2008 Chief Executive’s introduction Governance People Our true performance as a company must take account of both our non-financial and financial results. They are intrinsically linked – if we want to be a profitable and successful company, we must be an ethical one. We are committed to being both. To that end, we have been building on our expertise and engaging with stakeholders to find ways to innovate and improve. Health and safety In developing online solutions to the needs of our customers in areas like health, science and technology, law, environment, and business, we benefit society. For example, Procedures Consult gives doctors a way of maintaining their skills and knowledge through web-based simulation of essential medical techniques; TotalPatent is a single source for global patents, a primary driver in research and development; XpertHR helps practitioners advance good practice in people management; energylocate aggregates our energy offerings into a one-stop community, using tools like social networking and video to advance knowledge. Customers Innovation is also helping advance our internal corporate responsibility: > Increased online training in our Code of Ethics and Business Conduct has led to more of our people understanding what it means to do the right thing > A new global jobs board illuminates our value, Boundarylessness, by allowing employees to search for new positions across locations, functions,