Properties and Characteristics of Graphite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Graphite Materials for the U.S
ANRIC your success is our goal SUBSECTION HH, Subpart A Timothy Burchell CNSC Contract No: 87055-17-0380 R688.1 Technical Seminar on Application of ASME Section III to New Materials for High Temperature Reactors Delivered March 27-28, 2018, Ottawa, Canada TIM BURCHELL BIOGRAPHICAL INFORMATION Dr. Tim Burchell is Distinguished R&D staff member and Team Lead for Nuclear Graphite in the Nuclear Material Science and Technology Group within the Materials Science and Technology Division of the Oak Ridge National Laboratory (ORNL). He is engaged in the development and characterization of carbon and graphite materials for the U.S. Department of Energy. He was the Carbon Materials Technology (CMT) Group Leader and was manager of the Modular High Temperature Gas-Cooled Reactor Graphite Program responsible for the research project to acquire reactor graphite property design data. Currently, Dr. Burchell is the leader of the Next Generation Nuclear Plant graphite development tasks at ORNL. His current research interests include: fracture behavior and modeling of nuclear-grade graphite; the effects of neutron damage on the structure and properties of fission and fusion reactor relevant carbon materials, including isotropic and near isotropic graphite and carbon-carbon composites; radiation creep of graphites, the thermal physical properties of carbon materials. As a Research Officer at Berkeley Nuclear Laboratories in the U.K. he monitored the condition of graphite moderators in gas-cooled power reactors. He is a Battelle Distinguished Inventor; received the Hsun Lee Lecture Award from the Chinese Academy of Science’s Institute of Metals Research in 2006 and the ASTM D02 Committee Eagle your success is our goal Award in 2015. -

The Era of Carbon Allotropes Andreas Hirsch
commentary The era of carbon allotropes Andreas Hirsch Twenty-five years on from the discovery of 60C , the outstanding properties and potential applications of the synthetic carbon allotropes — fullerenes, nanotubes and graphene — overwhelmingly illustrate their unique scientific and technological importance. arbon is the element in the periodic consist of extended networks of sp3- and 1985, with the advent of fullerenes (Fig. 1), table that provides the basis for life sp2 -hybridized carbon atoms, respectively. which were observed for the first time by Con Earth. It is also important for Both forms show unique physical properties Kroto et al.3. This serendipitous discovery many technological applications, ranging such as hardness, thermal conductivity, marked the beginning of an era of synthetic from drugs to synthetic materials. This lubrication behaviour or electrical carbon allotropes. Now, as we celebrate role is a consequence of carbon’s ability conductivity. Conceptually, many other buckminsterfullerene’s 25th birthday, it is to bind to itself and to nearly all elements ways to construct carbon allotropes are also the time to reflect on a growing family in almost limitless variety. The resulting possible by altering the periodic binding of synthetic carbon allotropes, which structural diversity of organic compounds motif in networks consisting of sp3-, sp2- includes the synthesis of carbon nanotubes and molecules is accompanied by a broad and sp-hybridized carbon atoms1,2. As a in 19914 and the rediscovery of graphene range of -

Chemistry with Graphene and Graphene Oxide - Challenges for Synthetic Chemists Siegfried Eigler* and Andreas Hirsch*
Chemistry with Graphene and Graphene Oxide - Challenges for Synthetic Chemists Siegfried Eigler* and Andreas Hirsch* Dr. Siegfried Eigler*, Prof. Dr. Andreas Hirsch* Department of Chemistry and Pharmacy and Institute of Advanced Materials and Processes (ZMP) Henkestrasse 42, 91054 Erlangen and Dr.-Mack Strasse 81, 90762 Fürth (Germany) Fax: (+49) 911-6507865015 E-mail: [email protected]; [email protected] The chemical production of graphene as well as its controlled wet- chemical modification is a challenge for synthetic chemists and the characterization of reaction products requires sophisticated analytic methods. In this review we first describe the structure of graphene and graphene oxide. We then outline the most important synthetic methods which are used for the production of these carbon based nanomaterials. We summarize the state-of-the-art for their chemical functionalization by non-covalent and covalent approaches. We put special emphasis on the differentiation of the terms graphite, graphene, graphite oxide and graphene oxide. An improved fundamental knowledge about the structure and the chemical properties of graphene and graphene oxide is an important prerequisite for the development of practical applications. 1. Introduction: Graphene and Graphene Oxide – Opportunities and Challenges for Synthetic Chemists Research into graphene and graphene oxide (GO) represents an emerging field of interdisciplinary science spanning a variety of disciplines including chemistry, physics, materials science, device fabrication and -

Compressive Behavior Characteristics of High-Performance Slurry-Infiltrated Fiber-Reinforced Cementitious Composites (Sifrccs) Under Uniaxial Compressive Stress
materials Article Compressive Behavior Characteristics of High-Performance Slurry-Infiltrated Fiber-Reinforced Cementitious Composites (SIFRCCs) under Uniaxial Compressive Stress Seungwon Kim 1 , Seungyeon Han 2, Cheolwoo Park 1,* and Kyong-Ku Yun 2,* 1 Department of Civil Engineering, Kangwon National University, 346 Jungang-ro, Samcheok 25913, Korea; [email protected] 2 KIIT (Kangwon Institute of Inclusive Technology), Kangwon National University, 1 Gangwondaegil, Chuncheon 24341, Korea; [email protected] * Correspondence: [email protected] (C.P.); [email protected] (K.-K.Y.); Tel.: +82-33-570-6515 (C.P.); +82-33-250-6236 (K.-K.Y.) Received: 11 October 2019; Accepted: 19 December 2019; Published: 1 January 2020 Abstract: The compressive stress of concrete is used as a design variable for reinforced concrete structures in design standards. However, as the performance-based design is being used with increasing varieties and strengths of concrete and reinforcement bars, mechanical properties other than the compressive stress of concrete are sometimes used as major design variables. In particular, the evaluation of the mechanical properties of concrete is crucial when using fiber-reinforced concrete. Studies of high volume fractions in established compressive behavior prediction equations are insufficient compared to studies of conventional fiber-reinforced concrete. Furthermore, existing prediction equations for the mechanical properties of high-performance fiber-reinforced cementitious composite and high-strength concrete have limitations in terms of the strength and characteristics of contained fibers (diameter, length, volume fraction) even though the stress-strain relationship is determined by these factors. Therefore, this study developed a high-performance slurry-infiltrated fiber-reinforced cementitious composite that could prevent the fiber ball phenomenon, a disadvantage of conventional fiber-reinforced concrete, and maximize the fiber volume fraction. -

Coal Characteristics
CCTR Indiana Center for Coal Technology Research COAL CHARACTERISTICS CCTR Basic Facts File # 8 Brian H. Bowen, Marty W. Irwin The Energy Center at Discovery Park Purdue University CCTR, Potter Center, 500 Central Drive West Lafayette, IN 47907-2022 http://www.purdue.edu/dp/energy/CCTR/ Email: [email protected] October 2008 1 Indiana Center for Coal Technology Research CCTR COAL FORMATION As geological processes apply pressure to peat over time, it is transformed successively into different types of coal Source: Kentucky Geological Survey http://images.google.com/imgres?imgurl=http://www.uky.edu/KGS/coal/images/peatcoal.gif&imgrefurl=http://www.uky.edu/KGS/coal/coalform.htm&h=354&w=579&sz= 20&hl=en&start=5&um=1&tbnid=NavOy9_5HD07pM:&tbnh=82&tbnw=134&prev=/images%3Fq%3Dcoal%2Bphotos%26svnum%3D10%26um%3D1%26hl%3Den%26sa%3DX 2 Indiana Center for Coal Technology Research CCTR COAL ANALYSIS Elemental analysis of coal gives empirical formulas such as: C137H97O9NS for Bituminous Coal C240H90O4NS for high-grade Anthracite Coal is divided into 4 ranks: (1) Anthracite (2) Bituminous (3) Sub-bituminous (4) Lignite Source: http://cc.msnscache.com/cache.aspx?q=4929705428518&lang=en-US&mkt=en-US&FORM=CVRE8 3 Indiana Center for Coal Technology Research CCTR BITUMINOUS COAL Bituminous Coal: Great pressure results in the creation of bituminous, or “soft” coal. This is the type most commonly used for electric power generation in the U.S. It has a higher heating value than either lignite or sub-bituminous, but less than that of anthracite. Bituminous coal -
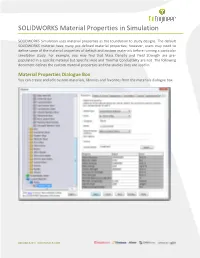
SOLIDWORKS Material Properties in Simulation
SOLIDWORKS Material Properties in Simulation SOLIDWORKS Simulation uses material properties as the foundation to study designs. The default SOLIDWORKS material have many pre-defined material properties; however, users may need to define some of the material properties of default and custom materials before running a particular simulation study. For example, you may find that Mass Density and Yield Strength are pre- populated in a specific material but Specific Heat and Thermal Conductivity are not. The following document defines the custom material properties and the studies they are used in. Material Properties Dialogue Box You can create and edit custom materials, libraries and favorites from the materials dialogue box. 888.688.3234 | GOENGINEER.COM Elastic Modulus The Elastic Modulus (Young’s Modulus) is the ratio of stress versus strain in the X, Y or Z directions. Elastic Moduli are used in static, nonlinear, frequency, dynamic, and buckling analyses. Poisson's Ratio Poisson’s Ratio is the negative ratio between transverse and axial strain. Poisson’s ratios are dimensionless quantities. For isotropic materials, the Poisson’s ratios in all planes are equal. Poisson ratios are used in static, nonlinear, frequency, dynamic and buckling Shear Modulus Shear Modulus (Modulus of Rigidity) is the ratio of shear stress to shear strain Shear Moduli are used in static, nonlinear, frequency, dynamic and buckling analyses. Mass Density Mass Density is used in static, nonlinear, frequency, dynamic, buckling, and thermal analyses. Static and buckling analyses use this property only if you define body forces (gravity and/or centrifugal). Tensile Strength Tensile Strength is the maximum that a material can withstand before stretching or breaking. -

The Strength of Concrete
The Strength of Chapter Concrete 3 3.1 The Importance of Strength 3.2 Strength Level Required KINDS OF STRENGTH 3.3 Compressive Strength 3.4 Flexural Strength 3.5 Tensile Strength 3.6 Shear, Torsion and Combined Stresses 3.7 Relationship of Test Strength to the Structure MEASUREMENT OF STRENGTH 3.8 Job-Molded Specimens 3.9 Te s t i n g o f H a r d e n e d C o n c r e t e FACTORS AFFECTING STRENGTH 3.10 General Comments 3.11 Causes of Strength Variations –Cement – Aggregates – Mix Proportioning – Making and Handling the Concrete – Temperature and Curing 3.12 Apparent Low Strength 3.13 Accelerated Strength Development – High-Early-Strength Cement –Admixtures – Retention of Heat of Hydration – High-Temperature Curing – Rapid-Setting Cements 3.14 Slow Strength Development HIGH-STRENGTH CONCRETE (HSC) 3.15 Selection of Materials and Mix 3.16 Handling and Quality Control EARLY MEASUREMENT OF STRENGTH EXPOSURE TO HIGH TEMPERATURE 3.17 Long-Time Exposure 3.18 Fire-Damaged Concrete 3 The Strength of Concrete The quality of concrete is judged largely on the strength of that concrete. Equipment and methods are continually being modernized, testing methods are improved, and means of analyzing and interpreting test data are becoming more sophisticated. Prior to the 2008 edition of the ACI 318 Standard, we relied almost exclusively on the strength of 6-by-12-inch cylinders, made on the jobsite and tested in compression at 28 days age for evaluation and acceptance of concrete. The use of 4-by-8-inch cylinders for strength evaluation was first addressed in ACI 318-08. -

Properties and Characteristics of Graphite
SPECIALTY MATERIALS PROPERTIES AND CHARACTERISTICS OF GRAPHITE For the semiconductor industry May 2013 INTRODUCTION Introduction Table of Contents As Entegris has continued to grow its market share of Introduction .............................................................1 manufactured graphite material and to expand their usage into increasingly complex areas, the need to Structure ..................................................................2 be more technically versed in both Entegris’ POCO® graphite and other manufactured graphite properties Apparent Density .....................................................6 and how they are tested has also grown. It is with this Porosity ....................................................................8 thought in mind that this primer on properties and characteristics of graphite was developed. Hardness ................................................................11 POCO, now owned by Entegris, has been a supplier Compressive Strength .............................................12 to the semiconductor industry for more than 35 years. Products include APCVD wafer carriers, E-Beam cruci- Flexural Strength ...................................................14 bles, heaters (small and large), ion implanter parts, Tensile Strength .....................................................16 LTO injector tubes, MOCVD susceptors, PECVD wafer trays and disk boats, plasma etch electrodes, quartz Modulus of Elasticity .............................................18 replacement parts, sealing -

Graphene: a Peculiar Allotrope of Carbon
Graphene: A Peculiar Allotrope Of Carbon Laxmi Nath Bhattarai Department of Physics, Butwal Multiple Campus Correspondence: [email protected] Abstract Graphene is a two dimensional one atom thick allotrope of Carbon. Electrons in grapheme behave as massless relativistic particles. It is a 2 dimensional nanomaterial with many peculiar properties. In grapheme both integral and fractional quantum Hall effects are observed. Many practical application are seen from use of Graphene material. Keywords: Graphene, allotrope, Quantum Hall effect, nanomaterial. Introduction Graphene is a two dimensional allotropic form of Honey comb structure of Graphene carbon. This was discovered in 2004. Diamond and Graphene is considered as the mother of all graphitic Graphite are three dimensional allotropes of carbon materials because it is the building block of carbon which are known from ancient time. One dimensional materials of all other dimensions (Srinivasan, 2007). carbon nanotubes were discovered in 1991 and Griphite is obtained by the stacking of Graphene zero dimensional Fullerenes were discovered in layers, Diamond can be obtained from Graphene under 1985. Graphene was experimentally extracted from extreme pressure and temperatures. One-dimensional 3-dimensional Graphite by Physisists Andre Geim and carbon nanotubes can be obtained by rolling and Konstantin Novoselov of Manchester University UK Zero–dimensional Fullerenes is obtained by wrapping in 2004. For their remarkable work they were awarded Graphene layers. the Nobel Prize of Physics for the year 2010. Properties Structure The young material Graphene is found to have Graphene is a mono layer of Carbon atoms packed following unique properties: into a honeycomb crystal structure. Graphene sheets 2 are one atom thick 2-dimensional layers of sp – bonded a) It is the thinnest material in the universe and the carbon. -

Comparison of Tensile and Compressive Properties of Carbon/Glass Interlayer and Intralayer Hybrid Composites
materials Article Comparison of Tensile and Compressive Properties of Carbon/Glass Interlayer and Intralayer Hybrid Composites Weili Wu 1 ID , Qingtao Wang 1 ID and Wei Li 1,2,3,* ID 1 College of Textiles, Donghua University, No. 2999, Northern Renmin Rd., Songjiang District, Shanghai 201620, China; [email protected] (W.W.); [email protected] (Q.W.) 2 Key Lab of Textile Science & Technology, Ministry of Education, Shanghai 201620, China 3 Center for Civil Aviation Composites, Shanghai 201620, China * Correspondence: [email protected]; Tel.: +86-137-6402-2421 Received: 27 May 2018; Accepted: 25 June 2018; Published: 28 June 2018 Abstract: Tensile and compressive properties of interlayer and intralayer hybrid composites were investigated in this paper. The tensile modulus and compression modulus of interlayer and intralayer hybrid composites are the same under the same mixed ratio, the tensile strength is much superior to the compression strength, and while the tensile modulus and strength increase along with the carbon fiber content, the compression values change slightly. The influence of stacking structures on the tensile and compressive strengths is opposite to the ratio of T/C (tensile/compression) strength for interlayer hybrid composites, and while the tensile and compression strengths with glass fiber sandwiching carbon fiber can reach the maximum value, the ratio of T/C strength is minimum. For structures with carbon fiber sandwiching glass fiber, or with asymmetric structures, the tensile and compressive strengths are at a low value. For intralayer hybrid structures, while the carbon/glass (C/G) dispersion degree is high, the tensile and compression strengths are low. -

The Mechanical Properties of Saline Ice Under Uniaxial Compression Gary A
IGS lntern•tional Symp<>Sium Ofl Applied (a! and Snow Re:sevd!:, Rovaniemi. Flnland; 18·23 April 1993 The Mechanical Properties of Saline Ice Under Uniaxial Compression Gary A. Kuehn and Briand M. Schulson Ice Research Laboratory Thayer School of Engineering, Dartmouth College 8000 Cummings Hall, Hanover, NH 03755-8000 603-646-3763, 603-646-3828 : FAX [email protected] : internet [email protected] : internet Annals of Glaciology ABSTRACT (in press, June 1993) Understanding the mechanical properties of saline ice is important for engineering design as well as for operations in polar regions. In order to gain understanding of the basic mechanisms of deformation and fracture, laboratory-grown columnar saline ice, representative of first-year sea ice, was tested in uniaxial comptession under a variety of conditions of strain rate oo-7 to 10-1 per second), temperature (-40', -20', -10· and .5·c) and orientation (loading vertically or horizontally: i.e. parallel or perpendicular to the growth direction). The range of strain rate spanned the ductile-to-brittle transition for every combination of temperature and specimen orientation. The results of over 250 tests are reported. Mechanical properties, failure mode and ice structure are analyzed with respect to the testing conditions. The results show that strength is dependent upon the ice structure, orientation, strain rate and temperature. During loading in the ductile regime the structure is altered (e.g. by recrystallization), whereas in the brittle regime the majority of the structural change is through cracking. The results are compared to results from the literature on both natural sea ice and laboratory-grown saline ice. -
Nuclear Graphite for High Temperature Reactors
Nuclear Graphite for High temperature Reactors B J Marsden AEA Technology Risley, Warrington Cheshire, WA3 6AT, UK Abstract The cores and reflectors in modem High Temperature Gas Cooled Reactors (HTRs) are constructed from graphite components. There are two main designs; the Pebble Bed design and the Prism design, see Table 1. In both of these designs the graphite not only acts as a moderator, but is also a major structural component that may provide channels for the fuel and coolant gas, channels for control and safety shut off devices and provide thermal and neutron shielding. In addition, graphite components may act as a heat sink or conduction path during reactor trips and transients. During reactor operation, many of the graphite component physical properties are significantly changed by irradiation. These changes lead to the generation of significant internal shrinkage stresses and thermal shut down stresses that could lead to component failure. In addition, if the graphite is irradiated to a very high irradiation dose, irradiation swelling can lead to a rapid reduction in modulus and strength, making the component friable. The irradiation behaviour of graphite is strongly dependent on its virgin microstructure, which is determined by the manufacturing route. Nevertheless, there are available, irradiation data on many obsolete graphites of known microstructures. There is also a well-developed physical understanding of the process of irradiation damage in graphite. This paper proposes a specification for graphite suitable for modem HTRs. HTR Graphite Component Design and Irradiation Environment The details of the HTRs, which have, or are being, been built and operated, are listed in Table 1.