Molecular Regulation of Forebrain Development and Neural Stem/Progenitor Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What to Expect After Having a Subarachnoid Hemorrhage (SAH) Information for Patients and Families Table of Contents
What to expect after having a subarachnoid hemorrhage (SAH) Information for patients and families Table of contents What is a subarachnoid hemorrhage (SAH)? .......................................... 3 What are the signs that I may have had an SAH? .................................. 4 How did I get this aneurysm? ..................................................................... 4 Why do aneurysms need to be treated?.................................................... 4 What is an angiogram? .................................................................................. 5 How are aneurysms repaired? ..................................................................... 6 What are common complications after having an SAH? ..................... 8 What is vasospasm? ...................................................................................... 8 What is hydrocephalus? ............................................................................... 10 What is hyponatremia? ................................................................................ 12 What happens as I begin to get better? .................................................... 13 What can I expect after I leave the hospital? .......................................... 13 How will the SAH change my health? ........................................................ 14 Will the SAH cause any long-term effects? ............................................. 14 How will my emotions be affected? .......................................................... 15 When should -

Meninges Ventricles And
Meninges ,ventricles & CSF Dr.Sanaa Al-Shaarawy Dr. Essam Eldin Salama OBJECTIVES • By the end of the lecture the student should be able to: • Describe the cerebral meninges & list the main dural folds. • Describe the spinal meninges & locate the level of the termination of each of them. • Describe the importance of the subarachnoid space. • List the Ventricular system of the CNS and locate the site of each of them. • Describe the formation, circulation, drainage, and functions of the CSF. • Know some clinical point about the CSF MENINGES • The brain and spinal cord are invested by three concentric membranes ; • The outermost layer is the dura matter. • The middle layer is the arachnoid matter. • The innermost layer is the pia matter. DURA MATER ▪The cranial dura is a two layered tough, fibrous thick membrane that surrounds the brain. ▪It is formed of two layers; periosteal and meningeal. ▪The periosteal layer is attached to the skull. ▪The meningeal layer is folded forming the dural folds : falx cerebri, and tentorium cerebelli. ▪Sensory innervation of the dura is mostly from : meningeal branches of the trigeminal and vagus nerves & C1 to C3(upper cervical Ns.). DURA MATER Folds Two large reflection of dura extend into the cranial cavity : 1.The falx cerebri, In the midline, ▪It is a vertical sickle-shaped sheet of dura, extends from the cranial roof into the great longitudinal fissure between the two cerebral hemispheres. ▪It has an attached border adherent to the skull. ▪And a free border lies above the corpus callosum. DURA MATER Folds 2. A horizontal shelf of dura, The tentorium cerebelli, ▪ It lies between the posterior part of the cerebral hemispheres and the cerebellum. -

Structure and Junctional Complexes of Endothelial, Epithelial and Glial Brain Barriers
International Journal of Molecular Sciences Review Structure and Junctional Complexes of Endothelial, Epithelial and Glial Brain Barriers Mariana Castro Dias *, Josephine A. Mapunda, Mykhailo Vladymyrov and Britta Engelhardt * Theodor Kocher Institute, University of Bern, 3012 Bern, Switzerland; [email protected] (J.A.M.); [email protected] (M.V.) * Correspondence: [email protected] (M.C.D.); [email protected] (B.E.) Received: 14 October 2019; Accepted: 26 October 2019; Published: 29 October 2019 Abstract: The homeostasis of the central nervous system (CNS) is ensured by the endothelial, epithelial, mesothelial and glial brain barriers, which strictly control the passage of molecules, solutes and immune cells. While the endothelial blood-brain barrier (BBB) and the epithelial blood-cerebrospinal fluid barrier (BCSFB) have been extensively investigated, less is known about the epithelial and mesothelial arachnoid barrier and the glia limitans. Here, we summarize current knowledge of the cellular composition of the brain barriers with a specific focus on describing the molecular constituents of their junctional complexes. We propose that the brain barriers maintain CNS immune privilege by dividing the CNS into compartments that differ with regard to their role in immune surveillance of the CNS. We close by providing a brief overview on experimental tools allowing for reliable in vivo visualization of the brain barriers and their junctional complexes and thus the respective CNS compartments. Keywords: brain barriers; blood-brain barrier; neurovascular unit; blood-cerebrospinal fluid barrier; arachnoid barrier; glia limitans; tight junctions; adherens junctions 1. Introduction The brain barriers established by the endothelial blood-brain barrier (BBB), the epithelial blood-cerebrospinal fluid barrier (BCSFB), the meningeal brain barriers and the blood spinal cord barrier are essential for maintaining central nervous system (CNS) homeostasis [1]. -

Lecture 4: the Meninges And
1/1/2016 Introduction • Protection of the brain – Bone (skull) The Nervous System – Membranes (meninges) – Watery cushion (cerebrospinal fluid) – Blood-brain barrier (astrocytes) Meninges CSF The Meninges The Meninges • Series of membranes • Three layers • Cover and protect the CNS – Dura mater • Anchor and cushion the brain – Arachnoid mater – • Contain cerebrospinal fluid (CSF) Pia mater The Meninges • Dura mater – “Tough mother” Skin of scalp Periosteum – Strongest meninx Bone of skull Periosteal Dura – Fibrous connective tissue Meningeal mater Superior Arachnoid mater – sagittal sinus Pia mater Limit excessive movement of the brain Subdural Arachnoid villus – space Blood vessel Forms partitions in the skull Subarachnoid Falx cerebri space (in longitudinal fissure only) Figure 12.24 1 1/1/2016 Superior The Meninges sagittal sinus Falx cerebri • Arachnoid mater – “Spider mother” Straight sinus – Middle layer with weblike extensions Crista galli – Separated from the dura mater by the subdural space of the Tentorium ethmoid cerebelli – Subarachnoid space contains CSF and blood vessels bone Falx Pituitary cerebelli gland (a) Dural septa Figure 12.25a The Meninges • Pia mater – “Gentle mother” – Connected to the dura mater by projections from the arachnoid mater – Layer of delicate vascularized connective tissue – Clings tightly to the brain T Meningitis TT121212 Ligamentum flavumflavumflavum L • LL555 Lumbar puncture Inflammation of meninges needle entering subarachnoid • May be bacterial or viral spacespacespace LLL444 • Diagnosed by -

Subarachnoid Trabeculae: a Comprehensive Review of Their Embryology, Histology, Morphology, and Surgical Significance Martin M
Literature Review Subarachnoid Trabeculae: A Comprehensive Review of Their Embryology, Histology, Morphology, and Surgical Significance Martin M. Mortazavi1,2, Syed A. Quadri1,2, Muhammad A. Khan1,2, Aaron Gustin3, Sajid S. Suriya1,2, Tania Hassanzadeh4, Kian M. Fahimdanesh5, Farzad H. Adl1,2, Salman A. Fard1,2, M. Asif Taqi1,2, Ian Armstrong1,2, Bryn A. Martin1,6, R. Shane Tubbs1,7 Key words - INTRODUCTION: Brain is suspended in cerebrospinal fluid (CSF)-filled sub- - Arachnoid matter arachnoid space by subarachnoid trabeculae (SAT), which are collagen- - Liliequist membrane - Microsurgical procedures reinforced columns stretching between the arachnoid and pia maters. Much - Subarachnoid trabeculae neuroanatomic research has been focused on the subarachnoid cisterns and - Subarachnoid trabecular membrane arachnoid matter but reported data on the SAT are limited. This study provides a - Trabecular cisterns comprehensive review of subarachnoid trabeculae, including their embryology, Abbreviations and Acronyms histology, morphologic variations, and surgical significance. CSDH: Chronic subdural hematoma - CSF: Cerebrospinal fluid METHODS: A literature search was conducted with no date restrictions in DBC: Dural border cell PubMed, Medline, EMBASE, Wiley Online Library, Cochrane, and Research Gate. DL: Diencephalic leaf Terms for the search included but were not limited to subarachnoid trabeculae, GAG: Glycosaminoglycan subarachnoid trabecular membrane, arachnoid mater, subarachnoid trabeculae LM: Liliequist membrane ML: Mesencephalic leaf embryology, subarachnoid trabeculae histology, and morphology. Articles with a PAC: Pia-arachnoid complex high likelihood of bias, any study published in nonpopular journals (not indexed PPAS: Potential pia-arachnoid space in PubMed or MEDLINE), and studies with conflicting data were excluded. SAH: Subarachnoid hemorrhage SAS: Subarachnoid space - RESULTS: A total of 1113 articles were retrieved. -

Spinal Meninges Neuroscience Fundamentals > Regional Neuroscience > Regional Neuroscience
Spinal Meninges Neuroscience Fundamentals > Regional Neuroscience > Regional Neuroscience SPINAL MENINGES GENERAL ANATOMY Meningeal Layers From outside to inside • Dura mater • Arachnoid mater • Pia mater Meningeal spaces From outside to inside • Epidural (above the dura) - See: epidural hematoma and spinal cord compression from epidural abscess • Subdural (below the dura) - See: subdural hematoma • Subarachnoid (below the arachnoid mater) - See: subarachnoid hemorrhage Spinal canal Key Anatomy • Vertebral body (anteriorly) • Vertebral arch (posteriorly). • Vertebral foramen within the vertebral arch. MENINGEAL LAYERS 1 / 4 • Dura mater forms a thick ring within the spinal canal. • The dural root sheath (aka dural root sleeve) is the dural investment that follows nerve roots into the intervertebral foramen. • The arachnoid mater runs underneath the dura (we lose sight of it under the dural root sheath). • The pia mater directly adheres to the spinal cord and nerve roots, and so it takes the shape of those structures. MENINGEAL SPACES • The epidural space forms external to the dura mater, internal to the vertebral foramen. • The subdural space lies between the dura and arachnoid mater layers. • The subarachnoid space lies between the arachnoid and pia mater layers. CRANIAL VS SPINAL MENINGES  Cranial Meninges • Epidural is a potential space, so it's not a typical disease site unless in the setting of high pressure middle meningeal artery rupture or from traumatic defect. • Subdural is a potential space but bridging veins (those that pass from the subarachnoid space into the dural venous sinuses) can tear, so it is a common site of hematoma. • Subarachnoid space is an actual space and is a site of hemorrhage and infection, for example. -
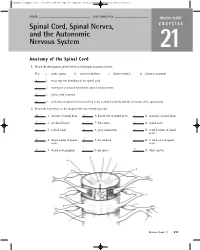
Spinal Cord, Spinal Nerves, and the Autonomic Nervous System
ighapmLre21pg211_216 5/12/04 2:24 PM Page 211 impos03 302:bjighapmL:ighapmLrevshts:layouts: NAME ___________________________________ LAB TIME/DATE _______________________ REVIEW SHEET Spinal Cord, Spinal Nerves, exercise and the Autonomic Nervous System 21 Anatomy of the Spinal Cord 1. Match the descriptions given below to the proper anatomical term: Key: a. cauda equina b. conus medullaris c. filum terminale d. foramen magnum d 1. most superior boundary of the spinal cord c 2. meningeal extension beyond the spinal cord terminus b 3. spinal cord terminus a 4. collection of spinal nerves traveling in the vertebral canal below the terminus of the spinal cord 2. Match the key letters on the diagram with the following terms. m 1. anterior (ventral) hornn 6. dorsal root of spinal nervec 11. posterior (dorsal) horn k 2. arachnoid materj 7. dura materf 12. spinal nerve a 3. central canalo 8. gray commissure i 13. ventral ramus of spinal nerve h 4. dorsal ramus of spinald 9. lateral horne 14. ventral root of spinal nerve nerve g l 5. dorsal root ganglion 10. pia materb 15. white matter o a b n c m d e l f g k h j i Review Sheet 21 211 ighapmLre21pg211_216 5/12/04 2:24 PM Page 212 impos03 302:bjighapmL:ighapmLrevshts:layouts: 3. Choose the proper answer from the following key to respond to the descriptions relating to spinal cord anatomy. Key: a. afferent b. efferent c. both afferent and efferent d. association d 1. neuron type found in posterior hornb 4. fiber type in ventral root b 2. -

The Meninges and Common Pathology Understanding the Anatomy Can Lead to Prompt Identification of Serious Pathology
education The meninges and common pathology Understanding the anatomy can lead to prompt identification of serious pathology The meninges are three membranous of the skull and extends into folds that arterial blood has sufficient pressure to layers that surround the structures of the compartmentalise the skull.1 2 The large separate the dura from the bare bone of central nervous system. They include the midline fold separates the two hemispheres the skull.4 The classic example of this is dura mater, the arachnoid mater, and and is called the falx.1 A smaller fold a severe blow to the temple that ruptures the pia mater. Together they cushion the separates the cerebral hemispheres from the middle meningeal artery, which brain and spinal cord with cerebrospinal the cerebellum and is known as the has part of its course between the skull fluid and support the associated vascular tentorium cerebelli usually abbreviated as and the dura at a weak point called the structures.1 2 Although they are usually “tentorium” (fig 1).1‑3 pterion.1 2 4 This creates an extradural mentioned as a trio, there are subtle but Where the edges of the falx and tentorium haematoma,2 a potentially lifethreatening important differences to the arrangement of meet the skull, the dura encloses large injury that classically presents with the meninges in the spine and cranium. The venous sinuses that are responsible for decreased consciousness and vomiting aim of this introduction to the meninges is draining venous blood from the brain.1 4 after a lucid interval (an initial period of to clarify the anatomy and link these details These are not to be confused with the air apparently normal consciousness). -

Skull, Brain and Cranial Nerves
Skull, Brain and Cranial Nerves Head and Neck Continued Skull Part of Axial Skeleton Cranial bones = cranium Enclose and protect brain Attachment for head + neck muscles pg 149 Facial bones =framework of face Form cavities for sense organs Opening for air + food passage Hold teeth Anchor face muscles Bones of Skull Flat bones: thin, flattened, some curve Sutures: immovable joints joining bones Calvaria = Skullcap =Vault Superior, Lateral, Posterior part of skull Floor = Base Inferior part of skull 85 openings in skull Spinal cord, blood vessels, nerves Cranial Fossae Created by bony ridges Supports, encircles brain 3 Fossae Anterior Middle Posterior Other small cavities in skull Middle Ear, Inner Ear Nasal Orbit pg 153 Skull through Life Ossifies late in 2nd month of development Frontal + Mandible start as 2 halves-then fuse Skull bones separated by unossified membranes = Fontanels Allow compression of skull during delivery Mostly replaced w/bone after 1st year Growth of Skull ½ adult size by age 9 months ¾ adult size by 2 years 100% adult size by 8-9 years Face enlarges between ages 6-13 years The Brain 4 Parts Cerebrum Diencephalon Brain Stem Pons Medulla Midbrain Cerebellum Gray matter surrounded by White matter pg 348 Meninges: 3 membranes around brain and spinal cord Made of Connective tissue Functions Cover, Protect CNS Enclose, protect blood vessels supplying CNS Contain CSF 3 Layers Dura Mater (external) Arachnoid Mater (middle) pg 375 Pia Mater (internal) Meninges (continued) Dura mater Strongest, -

Fig. 13.1 Copyright © Mcgraw-Hill Education
Fig. 13.1 Copyright © McGraw-Hill Education. Permission required for reproduction or display. C1 Cervical Cervical enlargement spinal nerves C7 Dural sheath Subarachnoid space Thoracic spinal Spinal cord nerves Vertebra (cut) Lumbar Spinal nerve enlargement T12 Spinal nerve rootlets Medullary cone Posterior median sulcus Lumbar Subarachnoid space Cauda equina spinal nerves Epidural space Posterior root ganglion L5 Rib Arachnoid mater Terminal Sacral Dura mater filum spinal nerves S5 Col (b) (a) 1 Fig. 13.2 Copyright © McGraw-Hill Education. Permission required for reproduction or display. Posterior Spinous process of vertebra Meninges: Dura mater (dural sheath) Arachnoid mater Fat in epidural space Pia mater Subarachnoid space Spinal cord Denticulate ligament Posterior root ganglion Spinal nerve Vertebral body (a) Spinal cord and vertebra (cervical) Anterior Posterior Gray matter: Central canal median sulcus White matter: Posterior horn Posterior column Gray commissure Lateral column Lateral horn Anterior column Anterior horn Posterior root of spinal nerve Posterior root ganglion Spinal nerve Anterior median fissure Anterior root of spinal nerve Meninges: Pia mater Arachnoid mater Dura mater (dural sheath) (b) Spinal cord and meninges (thoracic) (c) Lumbar spinal cord c: ©Ed Reschke/Getty Images 2 Table 13.1 3 Fig. 13.4 Copyright © McGraw-Hill Education. Permission required for reproduction or display. Ascending Descending tracts tracts Posterior column: Gracile fasciculus Cuneate fasciculus Anterior corticospinal tract Lateral Posterior spinocerebellar tract corticospinal tract Lateral reticulospinal tract Anterior spinocerebellar tract Tectospinal tract Anterolateral system (containing Medial reticulospinal tract spinothalamic and spinoreticular tracts) Lateral vestibulospinal tract Medial vestibulospinal tract 4 Fig. 13.5 Copyright © McGraw-Hill Education. Permission required for reproduction or display. -

The Spinal Cord and Spinal Nerves
14 The Nervous System: The Spinal Cord and Spinal Nerves PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The Central Nervous System (CNS) consists of: • The spinal cord • Integrates and processes information • Can function with the brain • Can function independently of the brain • The brain • Integrates and processes information • Can function with the spinal cord • Can function independently of the spinal cord © 2012 Pearson Education, Inc. Gross Anatomy of the Spinal Cord • Features of the Spinal Cord • 45 cm in length • Passes through the foramen magnum • Extends from the brain to L1 • Consists of: • Cervical region • Thoracic region • Lumbar region • Sacral region • Coccygeal region © 2012 Pearson Education, Inc. Gross Anatomy of the Spinal Cord • Features of the Spinal Cord • Consists of (continued): • Cervical enlargement • Lumbosacral enlargement • Conus medullaris • Cauda equina • Filum terminale: becomes a component of the coccygeal ligament • Posterior and anterior median sulci © 2012 Pearson Education, Inc. Figure 14.1a Gross Anatomy of the Spinal Cord C1 C2 Cervical spinal C3 nerves C4 C5 C 6 Cervical C 7 enlargement C8 T1 T2 T3 T4 T5 T6 T7 Thoracic T8 spinal Posterior nerves T9 median sulcus T10 Lumbosacral T11 enlargement T12 L Conus 1 medullaris L2 Lumbar L3 Inferior spinal tip of nerves spinal cord L4 Cauda equina L5 S1 Sacral spinal S nerves 2 S3 S4 S5 Coccygeal Filum terminale nerve (Co1) (in coccygeal ligament) Superficial anatomy and orientation of the adult spinal cord. The numbers to the left identify the spinal nerves and indicate where the nerve roots leave the vertebral canal. -

Meninges,Cerebrospinal Fluid, and the Spinal Cord
Homework PreLab 3 HW 3-4: Spinal Nerve Plexus Organization The Nervous System SPINAL CORD The Spinal Cord Continuation of CNS inferior to foramen magnum Simpler Conducts impulses to and from brain Two way conduction pathway Reflex actions The Spinal Cord Passes through vertebral canal Foramen magnum L2 Conus medullaris Filum terminale Cauda equina Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. The Spinal Cord Spinal nerves 31 pairs Cervical and lumbar enlargements Nerves serving the upper & lower limbs emerge here Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a The Spinal Cord Protection Bone Meninges CSF Spinal tap-inferior to L2 vertebra T12 Ligamentum flavum L5 Lumbar puncture needle entering subarachnoid space L4 Supra- spinous ligament L5 Filum terminale S1 Inter- Cauda equina vertebral Arachnoid Dura in subarachnoid disc matter mater space Figure 12.30 The Spinal Cord Cross section Central gray