Cayman NPS Metabolism Monograph Nathan K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug and Alcohol Withdrawal Clinical Practice Guidelines - NSW
Guideline Drug and Alcohol Withdrawal Clinical Practice Guidelines - NSW Summary To provide the most up-to-date knowledge and current level of best practice for the treatment of withdrawal from alcohol and other drugs such as heroin, and other opioids, benzodiazepines, cannabis and psychostimulants. Document type Guideline Document number GL2008_011 Publication date 04 July 2008 Author branch Centre for Alcohol and Other Drugs Branch contact (02) 9424 5938 Review date 18 April 2018 Policy manual Not applicable File number 04/2766 Previous reference N/A Status Active Functional group Clinical/Patient Services - Pharmaceutical, Medical Treatment Population Health - Pharmaceutical Applies to Area Health Services/Chief Executive Governed Statutory Health Corporation, Board Governed Statutory Health Corporations, Affiliated Health Organisations, Affiliated Health Organisations - Declared Distributed to Public Health System, Ministry of Health, Public Hospitals Audience All groups of health care workers;particularly prescribers of opioid treatments Secretary, NSW Health Guideline Ministry of Health, NSW 73 Miller Street North Sydney NSW 2060 Locked Mail Bag 961 North Sydney NSW 2059 Telephone (02) 9391 9000 Fax (02) 9391 9101 http://www.health.nsw.gov.au/policies/ space space Drug and Alcohol Withdrawal Clinical Practice Guidelines - NSW space Document Number GL2008_011 Publication date 04-Jul-2008 Functional Sub group Clinical/ Patient Services - Pharmaceutical Clinical/ Patient Services - Medical Treatment Population Health - Pharmaceutical -

(12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn Et Al
US 2004O224012A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn et al. (43) Pub. Date: Nov. 11, 2004 (54) TOPICAL APPLICATION AND METHODS Related U.S. Application Data FOR ADMINISTRATION OF ACTIVE AGENTS USING LIPOSOME MACRO-BEADS (63) Continuation-in-part of application No. 10/264,205, filed on Oct. 3, 2002. (76) Inventors: Pichit Suvanprakorn, Bangkok (TH); (60) Provisional application No. 60/327,643, filed on Oct. Tanusin Ploysangam, Bangkok (TH); 5, 2001. Lerson Tanasugarn, Bangkok (TH); Suwalee Chandrkrachang, Bangkok Publication Classification (TH); Nardo Zaias, Miami Beach, FL (US) (51) Int. CI.7. A61K 9/127; A61K 9/14 (52) U.S. Cl. ............................................ 424/450; 424/489 Correspondence Address: (57) ABSTRACT Eric G. Masamori 6520 Ridgewood Drive A topical application and methods for administration of Castro Valley, CA 94.552 (US) active agents encapsulated within non-permeable macro beads to enable a wider range of delivery vehicles, to provide longer product shelf-life, to allow multiple active (21) Appl. No.: 10/864,149 agents within the composition, to allow the controlled use of the active agents, to provide protected and designable release features and to provide visual inspection for damage (22) Filed: Jun. 9, 2004 and inconsistency. US 2004/0224012 A1 Nov. 11, 2004 TOPCAL APPLICATION AND METHODS FOR 0006 Various limitations on the shelf-life and use of ADMINISTRATION OF ACTIVE AGENTS USING liposome compounds exist due to the relatively fragile LPOSOME MACRO-BEADS nature of liposomes. Major problems encountered during liposome drug Storage in vesicular Suspension are the chemi CROSS REFERENCE TO OTHER cal alterations of the lipoSome compounds, Such as phos APPLICATIONS pholipids, cholesterols, ceramides, leading to potentially toxic degradation of the products, leakage of the drug from 0001) This application claims the benefit of U.S. -

Understanding Benzodiazephine Use, Abuse, and Detection
Siemens Healthcare Diagnostics, the leading clinical diagnostics company, is committed to providing clinicians with the vital information they need for the accurate diagnosis, treatment and monitoring of patients. Our comprehensive portfolio of performance-driven systems, unmatched menu offering and IT solutions, in conjunction with highly responsive service, is designed to streamline workflow, enhance operational efficiency and support improved patient care. Syva, EMIT, EMIT II, EMIT d.a.u., and all associated marks are trademarks of General Siemens Healthcare Diagnostics Inc. All Drugs other trademarks and brands are the Global Division property of their respective owners. of Abuse Siemens Healthcare Product availability may vary from Diagnostics Inc. country to country and is subject 1717 Deerfield Road to varying regulatory requirements. Deerfield, IL 60015-0778 Please contact your local USA representative for availability. www.siemens.com/diagnostics Siemens Global Headquarters Global Siemens Healthcare Headquarters Siemens AG Understanding Wittelsbacherplatz 2 Siemens AG 80333 Muenchen Healthcare Sector Germany Henkestrasse 127 Benzodiazephine Use, 91052 Erlangen Germany Abuse, and Detection Telephone: +49 9131 84 - 0 www.siemens.com/healthcare www.usa.siemens.com/diagnostics Answers for life. Order No. A91DX-0701526-UC1-4A00 | Printed in USA | © 2009 Siemens Healthcare Diagnostics Inc. Syva has been R1 R2 a leading developer N and manufacturer of AB R3 X N drugs-of-abuse tests R4 for more than 30 years. R2 C Now part of Siemens Healthcare ® Diagnostics, Syva boasts a long and Benzodiazepines have as their basic chemical structure successful track record in drugs-of-abuse a benzene ring fused to a seven-membered diazepine ring. testing, and leads the industry in the All important benzodiazepines contain a 5-aryl substituent ring (ring C) and a 1,4–diazepine ring. -

Risk Based Requirements for Medicines Handling
Risk based requirements for medicines handling Including requirements for Schedule 4 Restricted medicines Contents 1. Introduction 2 2. Summary of roles and responsibilities 3 3. Schedule 4 Restricted medicines 4 4. Medicines acquisition 4 5. Storage of medicines, including control of access to storage 4 5.1. Staff access to medicines storage areas 5 5.2. Storage of S4R medicines 5 5.3. Storage of S4R medicines for medical emergencies 6 5.4. Access to storage for S4R and S8 medicines 6 5.5. Pharmacy Department access, including after hours 7 5.6. After-hours access to S8 medicines in the Pharmacy Department 7 5.7. Storage of nitrous oxide 8 5.8. Management of patients’ own medicines 8 6. Distribution of medicines 9 6.1. Distribution outside Pharmacy Department operating hours 10 6.2. Distribution of S4R and S8 medicines 10 7. Administration of medicines to patients 11 7.1. Self-administration of scheduled medicines by patients 11 7.2. Administration of S8 medicines 11 8. Supply of medicines to patients 12 8.1. Supply of scheduled medicines to patients by health professionals other than pharmacists 12 9. Record keeping 13 9.1. General record keeping requirements for S4R medicines 13 9.2. Management of the distribution and archiving of S8 registers 14 9.3. Inventories of S4R medicines 14 9.4. Inventories of S8 medicines 15 10. Destruction and discards of S4R and S8 medicines 15 11. Management of oral liquid S4R and S8 medicines 16 12. Cannabis based products 17 13. Management of opioid pharmacotherapy 18 14. -

Socio-Demographic and Clinical Characteristics of Benzodiazepine Long-Term Users: Results from a Tertiary Care Center ⁎ F
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Florence Research Available online at www.sciencedirect.com ScienceDirect Comprehensive Psychiatry 69 (2016) 211–215 www.elsevier.com/locate/comppsych Socio-demographic and clinical characteristics of benzodiazepine long-term users: Results from a tertiary care center ⁎ F. Coscia, , G. Mansuetoa, M. Faccinib, R. Casarib, F. Lugobonib aDepartment of Health Sciences, University of Florence, via di San Salvi 12, 50135, Florence, Italy bAddiction Unit, Verona University Hospital, piazzale Aristide Stefani 1, 37126, Verona, Italy Abstract Objective: The use of benzodiazepines (BDZs) represents a critical issue since a long-term treatment may lead to dependence. This study aimed at evaluating socio-demographic and clinical characteristics of BZD long-term users who followed a detoxification program at a tertiary care center. Method: Two hundred-five inpatients were evaluated. Socio-demographic (e.g., gender, age, education) and clinical information (e.g., BZD used, dose, reason of prescription) was collected. BZDs dose was standardized as diazepam dose equivalents and was compared via the Defined Daily Dose (DDD). Chi-square, Fisher test, ANOVA and Bonferroni analyses were performed. Results: Females were more frequently BDZ long-term users than males. Hypnotic BZDs were frequently prescribed for problems different from sleep disturbances. Lorazepam, alprazolam, and lormetazepam were the most prescribed drugs. Lorazepam was more frequently used by males, consumed for a long period, in pills, and prescribed for anxiety. Lormetazepam was more frequently consumed by females with a high school education, having a psychiatric disorder, taken in drops and prescribed for insomnia. -

Table 6.12: Deaths from Poisoning, by Sex and Cause, Scotland, 2016
Table 6.12: Deaths from poisoning, by sex and cause, Scotland, 2016 ICD code(s), cause of death and substance(s) 1 Both Males Females ALL DEATHS FROM POISONING 2 1130 766 364 ACCIDENTS 850 607 243 X40 - X49 Accidental poisoning by and exposure to … X40 - Nonopioid analgesics, antipyretics and antirheumatics Paracetamol 2 1 1 Paracetamol, Cocaine, Amphetamine || 1 0 1 X41 - Antiepileptic, sedative-hypnotic, antiparkinsonism and psychotropic drugs, not elsewhere classified Alprazolam, MDMA, Cocaine || Cannabis, Alcohol 1 1 0 Alprazolam, Methadone || Pregabalin, Tramadol, Gabapentin, Cannabis 1 1 0 Alprazolam, Morphine, Heroin, Dihydrocodeine, Buprenorphine || Alcohol 1 1 0 Alprazolam, Oxycodone, Alcohol || Paracetamol 1 0 1 Amitriptyline, Cocaine, Etizolam || Paracetamol, Codeine, Hydrocodone, Alcohol 1 1 0 Amitriptyline, Dihydrocodeine || Diazepam, Paracetamol, Verapamil, Alcohol 1 1 0 Amitriptyline, Fluoxetine, Alcohol 1 0 1 Amitriptyline, Methadone, Diazepam || 1 1 0 Amitriptyline, Methadone, Morphine, Etizolam || Gabapentin, Cannabis, Alcohol 1 1 0 Amitriptyline, Venlafaxine 1 0 1 Amphetamine 1 1 0 Amphetamine || 1 1 0 Amphetamine || Alcohol 1 1 0 Amphetamine || Chlorpromazine 1 1 0 Amphetamine || Fluoxetine 1 1 0 Amphetamine, Dihydrocodeine, Alcohol || Procyclidine, Tramadol, Duloxetine, Haloperidol 1 0 1 Amphetamine, MDMA || Diclazepam, Cannabis, Alcohol 1 1 0 Amphetamine, Methadone || 1 1 0 Amphetamine, Oxycodone, Gabapentin, Zopiclone, Diazepam || Paracetamol, Alcohol 1 0 1 Amphetamine, Tramadol || Mirtazapine, Alcohol 1 1 0 Benzodiazepine -
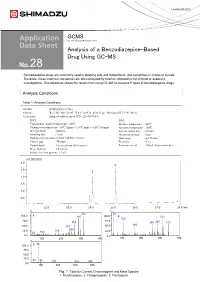
Analysis of a Benzodiazepine-Based Drug Using GC-MS 28
LAAN-E-MS-E028 GCMS Gas Chromatograph Mass Spectrometer Analysis of a Benzodiazepine-Based Drug Using GC-MS 28 Benzodiazepine drugs are commonly used in sleeping aids and tranquilizers, and sometimes in crimes or suicide. Therefore, these chemical substances are often analyzed by forensic laboratories for criminal or academic investigations. This datasheet shows the results from using GC-MS to measure 9 types of benzodiazepine drugs. Analysis Conditions Table 1: Analysis Conditions GC-MS :GCMS-QP2010 Ultra Column : Rxi®-5Sil MS (30 mL. X 0.25 mmI.D., df=0.25 µm, Shimadzu GLC P/N:13623) Glass insert :Silanized splitless insert (P/N: 221-48876-03) [GC] [MS] Vaporization chamber temperature : 260℃ Interface temperature : 280℃ Column oven temperature : 60℃ (2min) -> (10℃/min) -> 320℃ (10min) Ion source temperature : 200℃ Injection mode : Splitless Solvent elution time : 2.0 min Sampling time : 1 min Measurement mode : Scan High pressure injection method: 250 kPa (1.5 min) Mass range : m/z 35-600 Carrier gas : Helium Event time : 0.3 sec Control mode :Linear velocity (45.6 cm/sec) Emission current : 150 µA (high sensitivity) Purge flow rate :3.0 ml/min Sample injection quantity :1.0 µL (x1,000,000) 3.0 3 2.5 2 2.0 1.5 1.0 1 0.5 22.0 23.0 24.0 25.0 26.0 27.0 28.0 min % % 312 55 100.0 1 285 100.0 2 313 109 75.0 75.0 266 259287 342 166 50.0 238 50.0 248 25.0 183 25.0 63 109 0.0 0.0 100 200 300 400 100 200 300 400 % 86 100.0 3 75.0 50.0 25.0 58 99 183 315 387 0.0 100 200 300 400 Fig. -

Benzodiazepine Group ELISA Kit
Benzodiazepine Group ELISA Kit Benzodiazepine Background Since their introduction in the 1960s, benzodiazepines have been widely prescribed for the treatment of anxiety, insomnia, muscle spasms, alcohol withdrawal, and seizure-prevention as they are depressants of the central nervous system. Despite the fact that they are highly effective for their intended use, benzodiazepines are prescribed with caution as they can be highly addictive. In fact, researchers at NIDA (National Institute on Drug Abuse) have shown that addiction for benzodiazepines is similar to that of opioids, cannabinoids, and GHB. Common street names of benzodiazepines include “Benzos” and “Downers”. The five most encountered benzodiazepines on the illicit market are alprazolam (Xanax), lorazepam (Ativan), clonazepam (Klonopin), diazepam (Valium), and temazepam (Restori). The method of abuse is typically oral or snorted in crushed form. The DEA notes a particularly high rate of abuse among heroin and cocaine abusers. Designer benzodiazepines are currently offered in online shops selling “research chemicals”, providing drug abusers an alternative to prescription-only benzodiazepines. Data defining pharmacokinetic parameters, drug metabolisms, and detectability in biological fluids is limited. This lack of information presents a challenge to forensic laboratories. Changes in national narcotics laws in many countries led to the control of (phenazepam and etizolam), which were marketed by pharmaceutical companies in some countries. With the control of phenazepam and etizolam, clandestine laboratories have begun researching and manufacturing alternative benzodiazepines as legal substitutes. Delorazepam, diclazepam, pyrazolam, and flubromazepam have emerged as compounds in this class of drugs. References Drug Enforcement Administration, Office of Diversion Control. “Benzodiazepines.” http://www.deadiversion.usdoj.gov/drugs_concern/benzo_1. -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

The Misuse of Benzodiazepines Among High-Risk Opioid Users in Europe
EMBARGO — 7 JUNE 7. 6. 2018 UPDATED 11:30 Central European Time/CET (10:30 Western European Time/WET/Lisbon) Proof - 28 May 2018 not for circulation PERSPECTIVES ON DRUGS The misuse of benzodiazepines among high-risk opioid users in Europe Benzodiazepines are a widely prescribed I Introduction group of medicines with a range of clinical uses that include treating Benzodiazepines have a range of clinical uses and are among the most commonly prescribed medicines globally. anxiety, insomnia and managing alcohol They are useful in the short-term treatment of anxiety and withdrawal. This group of medicines is insomnia, and in managing alcohol withdrawal (Medicines often misused by high-risk opioid users, and Healthcare Products Regulatory Agency, 2015). Like all medicines, benzodiazepines can produce side effects. They and this is associated with considerable may also be misused, which we define as use without a morbidity and mortality. This paper prescription from a medical practitioner or, if prescribed, when describes the impact of benzodiazepines they are used outside accepted medical practice or guidelines. misuse on the health and treatment of While the misuse of benzodiazepines has been identified high-risk opioid users. as a concern for large groups in the general population, for example, among elderly people and women, this analysis focuses specifically on misuse among high-risk opioid users (1), a group of people among whom these medicines have been linked with severe treatment challenges and implicated in considerable numbers of drug-related deaths. It is important to stress that much benzodiazepine prescribing to high-risk drug users is done with legitimate therapeutic aims in mind. -

Indicators of Drug Or Alcohol Abuse Or Misuse
New Trends in Substance Abuse 2018 Lynn Riemer Ron Holmes, MD 720-480-0291 720-630-5430 [email protected] [email protected] www.actondrugs.org Facebook/ACTonDrugs Indicators of Drug or Alcohol Abuse or Misuse: Behavioral Physical - Abnormal behavior - Breath or body odor - Exaggerated behavior - Lack of coordination - Boisterous or argumentative - Uncoordinated & unsteady gait - Withdrawn - Unnecessary use of arms or supports for balance - Avoidance - Sweating and/or dry mouth - Changing emotions & erratic behavior - Change in appearance Speech Performance - Slurred or slow speech - Inability to concentrate - Nonsensical patterns - Fatigue & lack of motivation - Confusion - Slowed reactions - Impaired driving ability The physiologic factors predisposing to addiction Nearly every addictive drug targets the brain’s reward system by flooding the circuit with the neurotransmitter, dopamine. Neurotransmitters are necessary to transfer impulses from one brain cell to another. The brain adapts to the overwhelming surges in dopamine by ultimately producing less dopamine and by reducing the number of dopamine receptors in the reward circuit. As a result, two important physiologic adaptations occur: (1) the addict’s ability to enjoy the things that previously brought pleasure is impaired because of decreased dopamine, and (2) higher and higher doses of the abused drug are needed to achieve the same “high” that occurred when the drug was first used. This compels the addict to increase drug consumption to increase dopamine production leading to physiologic addiction with more and more intense cravings for the drug. The effects of addiction on the brain Nearly all substances of abuse affect the activity of neurotransmitters that play an important role in connecting one brain cell to another. -

ETIZOLAM Critical Review Report Agenda Item 4.13
ETIZOLAM Critical Review Report Agenda Item 4.13 Expert Committee on Drug Dependence Thirty-ninth Meeting Geneva, 6-10 November 2017 39th ECDD (2017) Agenda item 4.13 Etizolam Page 2 of 20 39th ECDD (2017) Agenda item 4.13 Etizolam Contents Acknowledgements.................................................................................................................................. 5 Summary...................................................................................................................................................... 6 1. Substance identification ....................................................................................................................... 7 A. International Nonproprietary Name (INN).......................................................................................................... 7 B. Chemical Abstract Service (CAS) Registry Number .......................................................................................... 7 C. Other Chemical Names ................................................................................................................................................... 7 D. Trade Names ....................................................................................................................................................................... 7 E. Street Names ....................................................................................................................................................................... 8 F. Physical Appearance