The Use of Bowel in Urologic Reconstructive Surgery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Anatomy of the Rectum and Anal Canal
BASIC SCIENCE identify the rectosigmoid junction with confidence at operation. The anatomy of the rectum The rectosigmoid junction usually lies approximately 6 cm below the level of the sacral promontory. Approached from the distal and anal canal end, however, as when performing a rigid or flexible sigmoid- oscopy, the rectosigmoid junction is seen to be 14e18 cm from Vishy Mahadevan the anal verge, and 18 cm is usually taken as the measurement for audit purposes. The rectum in the adult measures 10e14 cm in length. Abstract Diseases of the rectum and anal canal, both benign and malignant, Relationship of the peritoneum to the rectum account for a very large part of colorectal surgical practice in the UK. Unlike the transverse colon and sigmoid colon, the rectum lacks This article emphasizes the surgically-relevant aspects of the anatomy a mesentery (Figure 1). The posterior aspect of the rectum is thus of the rectum and anal canal. entirely free of a peritoneal covering. In this respect the rectum resembles the ascending and descending segments of the colon, Keywords Anal cushions; inferior hypogastric plexus; internal and and all of these segments may be therefore be spoken of as external anal sphincters; lymphatic drainage of rectum and anal canal; retroperitoneal. The precise relationship of the peritoneum to the mesorectum; perineum; rectal blood supply rectum is as follows: the upper third of the rectum is covered by peritoneum on its anterior and lateral surfaces; the middle third of the rectum is covered by peritoneum only on its anterior 1 The rectum is the direct continuation of the sigmoid colon and surface while the lower third of the rectum is below the level of commences in front of the body of the third sacral vertebra. -
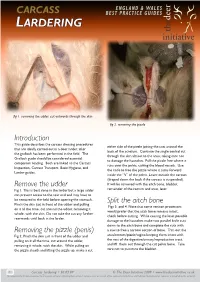
Introduction Remove the Udder Removing the Pizzle (Penis)
fig . removing the udder, cut outwards through the skin fig 2. removing the pizzle Introduction This guide describes the carcass dressing procedures either side of the pizzle joining the cuts around the that are ideally carried out in a deer larder, after back of the scrotum. Continue the single central cut the gralloch has been performed in the field. The through the skin almost to the anus, taking care not Gralloch guide should be considered essential to damage the haunches. Pull the pizzle free where it companion reading. Both are linked to the Carcass runs over the pelvis, cutting the blood vessels. Use Inspection, Carcass Transport, Basic Hygiene, and the knife to free the pizzle where it turns forward Larder guides. inside the “V” of the pelvis. Leave outside the carcass (draped down the back if the carcass is suspended). Remove the udder It will be removed with the aitch bone, bladder, Fig 1. This is best done in the larder but a large udder remainder of the rectum and anus, later. can prevent access to the rear end and may have to be removed in the field before opening the stomach. Split the aitch bone Pinch the skin just in front of the udder and pulling Figs 3. and 4. Note that some venison processors on it all the time, cut around the udder, removing it would prefer that the aitch bone remains intact, whole, with the skin. Do not take the cut any further check before cutting. While causing the least possible rearwards until back in the larder. -

Continent Urostomy Guide
$POUJOFOU6SPTUPNZ(VJEF "QVCMJDBUJPOPGUIF6OJUFE0TUPNZ"TTPDJBUJPOTPG"NFSJDB *OD i4FJ[FUIF 0QQPSUVOJUZw CONTINENT UROSTOMY GUIDE Ilene Fleischer, MSN, RN, CWOCN, Author Patti Wise, BSN, RN, CWOCN, Author Reviewed by: Authors and Victoria A.Weaver, RN, MSN, CETN Revised 2009 by Barbara J. Hocevar, BSN,RN,CWOCN, Manager, ET/WOC Nursing, Cleveland Clinic © 1985 Ilene Fleischer and Patti Wise This guidebook is available for free, in electronic form, from United Ostomy Associations of America (UOAA). UOAA may be contacted at: www.ostomy.org • [email protected] • 800-826-0826 CONTENTS INTRODUCTION . 3 WHAT IS A CONTINENT UROSTOMY? . 4 THE URINARY TRACT . 4 BEFORE THE SURGERY . .5 THE SURGERY . .5 THE STOMA . 7 AFTER THE SURGERY . 7 Irrigation of the catheter(s) 8 Care of the drainage receptacles 9 Care of the stoma 9 Other important information 10 ROUTINE CARE AT HOME . 10 Catheterization schedule 11 How to catheterize your pouch 11 Special considerations when catheterizing 11 Care of the catheter 12 Other routine care 12 HELPFUL HINTS . .13 SUPPLIES FOR YOUR CONTINENT UROSTOMY . 14 LIFE WITH YOUR CONTINENT UROSTOMY . 15 Clothing 15 Diet 15 Activity and exercise 15 Work 16 Travel 16 Telling others 17 Social relationships 17 Sexual relations and intimacy 17 RESOURCES . .19 GLOSSARY OF TERMS . 20 BIBLIOGRAPHY . .21 1 INTRODUCTION Many people have ostomies and lead full and active lives. Ostomy surgery is the main treatment for bypassing or replacing intestinal or urinary organs that have become diseased or dysfunctional. “Ostomy” means opening. It refers to a number of ways that bodily wastes are re-routed from your body. A urostomy specifi cally redirects urine. -

A Practical Guide for Stoma Problems Developed by the Ostomy Forum
A practical guide for Stoma problems Developed by the Ostomy Forum Dedicated to Stoma Care A practical guide for Stoma and Peristomal skin problems A practical guide for Stoma Developed by: Frances McKenzie, Amanda Smith, Doreen Woolley, Beverley Colton, Bart Tappe and Global Clinical Marketing, Dansac A/S. The practical guide is based on the Observation Index developed by the Ostomy Forum group (a specialized group of ET nurses from Sweden, Norway, The Netherlands, Poland, Japan, UK and Denmark) and is Normal Stoma made to help you manage common stoma and peristomal skin problems you might come across in your nursing practice. Stoma is a Greek word that means opening or mouth. It is a surgically created opening that can be temporary or permanent and allows for the Sharing best practice by use of this educational tool will lead to early excretion of faecal waste (colostomy, ileostomy) or urine (urostomy). detection and appropriate intervention to secure a high standard of stoma care. A stoma is a surgically made opening of the bowel: • The bowel is brought out through the abdominal wall This tool should be used in consultation with your Stoma Care Specialist. • It is matured and sutured subcutaneously • Faeces and urine will pass and be collected in a specially designed Disclaimer: ostomy pouch. We recognize that nurses in other practices will have different ways of treating the identified problems. The scope of this guide is to give first In the following pages you will find examples of different stoma problems step, easy to use, practical advice that is recognized and accepted and concrete suggestions for intervention and management of the stoma. -

How to Increase Your Enjoyment of Sex
BETTER SEX BETTER SEX BETTER SEX BETTER SEX BETTER SEX OF SEX ENJO YOUR INCREASE HOW TO for women and women for their partners YMENT “Sexual health is a state of physical, emotional, mental and social well-being in relation to sexuality; it is not merely the absence of disease, dysfunction or infirmity. Sexual health requires a positive and respectful approach to sexuality and sexual relationships, as well as the possibility of having pleasurable and safe sexual experiences, free of coercion, discrimination and violence. For sexual health to be attained and maintained, the sexual rights of all persons must be respected, protected and fulfilled.” Definition of sexual health, World Health Organisation SAFER SEX Using condoms for penetrative sex is the best way to protect yourself and your partners from Sexually Transmitted Infections, including HIV. Condoms also offer good protection from unwanted pregnancy. In the text of this booklet, we have chosen not to refer constantly to the use of condoms. Instead, we encourage you to make your own decisions about protecting yourself and others in each instance of sexual activity you undertake. 1 HOW TO INCREASE YOUR ENJOYMENT OF SEX This leaflet provides information on how to help yourself improve your enjoyment of sex. It has three main parts: Suggestions on how to improve sex generally, without doing formal exercises. These apply to both casual and regular partners. Exercises you can do on your own — for women who have difficulty getting turned on or experiencing orgasm, and who may or may not have a regular partner. Exercises you can do with a partner — for women who have difficulty getting turned on, or who have difficulty having an orgasm or enjoying penetrative sex. -

Adjustable Gastric Banding
7 Review Article Page 1 of 7 Adjustable gastric banding Emre Gundogdu, Munevver Moran Department of Surgery, Medical School, Istinye University, Istanbul, Turkey Contributions: (I) Conception and design: All authors; (II) Administrative support: All authors; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Emre Gündoğdu, MD, FEBS. Assistant Professor of Surgery, Department of Surgery, Medical School, Istinye University, Istanbul, Turkey. Email: [email protected]; [email protected]. Abstract: Gastric banding is based on the principle of forming a small volume pouch near the stomach by wrapping the fundus with various synthetic grafts. The main purpose is to limit oral intake. Due to the fact that it is a reversible surgery, ease of application and early results, the adjustable gastric band (AGB) operation has become common practice for the last 20 years. Many studies have shown that the effectiveness of LAGB has comparable results with other procedures in providing weight loss. Early studies have shown that short term complications after LAGB are particularly low when compared to the other complicated procedures. Even compared to RYGB and LSG, short-term results of LAGB have been shown to be significantly superior. However, as long-term results began to emerge, such as failure in weight loss, increased weight regain and long-term complication rates, interest in the procedure disappeared. The rate of revisional operations after LAGB is rapidly increasing today and many surgeons prefer to convert it to another bariatric procedure, such as RYGB or LSG, for revision surgery in patients with band removed after LAGB. -

Guidelines on Paediatric Urology S
Guidelines on Paediatric Urology S. Tekgül (Chair), H.S. Dogan, E. Erdem (Guidelines Associate), P. Hoebeke, R. Ko˘cvara, J.M. Nijman (Vice-chair), C. Radmayr, M.S. Silay (Guidelines Associate), R. Stein, S. Undre (Guidelines Associate) European Society for Paediatric Urology © European Association of Urology 2015 TABLE OF CONTENTS PAGE 1. INTRODUCTION 7 1.1 Aim 7 1.2 Publication history 7 2. METHODS 8 3. THE GUIDELINE 8 3A PHIMOSIS 8 3A.1 Epidemiology, aetiology and pathophysiology 8 3A.2 Classification systems 8 3A.3 Diagnostic evaluation 8 3A.4 Disease management 8 3A.5 Follow-up 9 3A.6 Conclusions and recommendations on phimosis 9 3B CRYPTORCHIDISM 9 3B.1 Epidemiology, aetiology and pathophysiology 9 3B.2 Classification systems 9 3B.3 Diagnostic evaluation 10 3B.4 Disease management 10 3B.4.1 Medical therapy 10 3B.4.2 Surgery 10 3B.5 Follow-up 11 3B.6 Recommendations for cryptorchidism 11 3C HYDROCELE 12 3C.1 Epidemiology, aetiology and pathophysiology 12 3C.2 Diagnostic evaluation 12 3C.3 Disease management 12 3C.4 Recommendations for the management of hydrocele 12 3D ACUTE SCROTUM IN CHILDREN 13 3D.1 Epidemiology, aetiology and pathophysiology 13 3D.2 Diagnostic evaluation 13 3D.3 Disease management 14 3D.3.1 Epididymitis 14 3D.3.2 Testicular torsion 14 3D.3.3 Surgical treatment 14 3D.4 Follow-up 14 3D.4.1 Fertility 14 3D.4.2 Subfertility 14 3D.4.3 Androgen levels 15 3D.4.4 Testicular cancer 15 3D.5 Recommendations for the treatment of acute scrotum in children 15 3E HYPOSPADIAS 15 3E.1 Epidemiology, aetiology and pathophysiology -

Renal Agenesis, Renal Tubular Dysgenesis, and Polycystic Renal Diseases
Developmental & Structural Anomalies of the Genitourinary Tract DR. Alao MA Bowen University Teach Hosp Ogbomoso Picture test Introduction • Congenital Anomalies of the Kidney & Urinary Tract (CAKUT) Objectives • To review the embryogenesis of UGS and dysmorphogenesis of CAKUT • To describe the common CAKUT in children • To emphasize the role of imaging in the diagnosis of CAKUT Introduction •CAKUT refers to gross structural anomalies of the kidneys and or urinary tract present at birth. •Malformation of the renal parenchyma resulting in failure of normal nephron development as seen in renal dysplasia, renal agenesis, renal tubular dysgenesis, and polycystic renal diseases. Introduction •Abnormalities of embryonic migration of the kidneys as seen in renal ectopy (eg, pelvic kidney) and fusion anomalies, such as horseshoe kidney. •Abnormalities of the developing urinary collecting system as seen in duplicate collecting systems, posterior urethral valves, and ureteropelvic junction obstruction. Introduction •Prevalence is about 3-6 per 1000 births •CAKUT is one of the commonest anomalies found in human. •It constitute approximately 20 to 30 percent of all anomalies identified in the prenatal period •The presence of CAKUT in a child raises the chances of finding congenital anomalies of other organ-systems Why the interest in CAKUT? •Worldwide, CAKUT plays a causative role in 30 to 50 percent of cases of end-stage renal disease (ESRD), •The presence of CAKUT, especially ones affecting the bladder and lower tract adversely affects outcome of kidney graft after transplantation Why the interest in CAKUT? •They significantly predispose the children to UTI and urinary calculi •They may be the underlying basis for urinary incontinence Genes & Environment Interact to cause CAKUT? • Tens of different genes with role in nephrogenesis have been identified. -

Complications of Urinary Diversion
Complications of Urinary Diversion Jennifer L. Dodson, M.D. Department of Urology Johns Hopkins University Types of Diversion Conduit Diversions Ileal conduit Colon conduit Continent Diversions Continent catheterizable reservoir Continent rectal pouch 1 Overview of Complications Mechanical Stoma problems Bowel obstruction Ureteral obstruction Reservoir perforation Metabolic Altered absorption Altered bone metabolism Growth delay Stones Cancer Conduit Diversions Ileal Conduit: Technically simplest Segment of choice Colon Conduit: Transverse or sigmoid Used when ileum not appropriate (eg: concomitant colon resection, abdominal radiation, short bowel syndrome, IBD) Early complications (< 30 days): 20-56% Late complications : 28-81% Risks: abdominal radiation abdominal surgery poor nutrition chronic steroids Farnham & Cookson, World J Urol, 2004 2 Complications of Ileal Conduit Campbell’s Urology, 8th Edition, 2002 Conduit: Bowel Complications Paralytic ileus 18-20% Conservative management vs NGT Consider TPN Bowel obstruction 5-10% Causes: Adhesions, internal hernia Evaluation: CT scan, Upper GI series Anastomotic leak 1-5 % Risk factors: bowel ischemia, radiation, steroids, IBD, technical error Prevention: Pre-operative bowel prep Attention to technical detail Stapled small-bowel Anastomosis (Campbell’s Blood supply, tension-free anastomosis, Urology, 8th Ed, 2004) realignment of mesentery Farnham & Cookson, World J Urol, 2004 3 Conduit Complications Conduit necrosis: Acute ischemia to bowel -
Hints and Tips
Hernia Simon, colostomy since 2010 Hints & Tips Dedicated to Stoma Care Dedicated to Stoma Care FOREWORD & ACKNOWLEDGEMENTS This booklet offers guidance to the person undergoing surgery which will result in stoma formation or for those post-operatively who may be at risk of or perhaps already have developed a parastomal hernia. Please discuss the content with your Stomal Therapy Nurse (STN) if you require additional advice or support. CONTENT What is a parastomal hernia? .........................................3 Am I at risk of developing a parastomal hernia? ������������4 What is my ideal weight? ................................................5 Practical hints & tips to reduce risk of developing a parastomal hernia .....................................6 I think I’ve developed a hernia - what should I do? ���������7 Parastomal hernia management �������������������������������������8 Exercise ..........................................................................9 Dansac would like to thank the following for their invaluable contribution to this booklet: Sharon Colman BSc (Hons) Community Stoma Nurse Specialist, Norfolk. Kevin Hayles Dip HE, RN Queens Hospital Romford, Essex. Debbie Johnson RGN, Stoma Specialist, Dansac UK, London Community. Jacqui North RGN BSc(Hons), Senior Clinical Nurse Specialist, Stoma Care, SE London Community. Jo Sica Clinical Nurse Specialist, Stoma Care, Kingston CCG. 2 WHAT IS A PARASTOMAL HERNIA? Parastomal hernia is a common complication which can affect some people following stoma formation. Research has shown that as many as 10-50% of patients may go on to develop a hernia.6, 9, 10 During your surgery an incision is made through the abdominal wall and muscle. This can result in a weakness in the muscle surrounding your stoma which may lead to a noticeable bulge behind or around the stoma. -

Bowel Function Anatomy
BOWEL FUNCTION ANATOMY Most of America gives little thought to bowel control. However, bowel control is actually a complex process involving the coordination of many different muscles and nerves. The bowel is considered to be a part of the digestive or gastrointestinal system. It is designed to help the body absorb nutrients and fluids from the foods we eat and drink. After taking out everything the body needs, the bowel then expels the leftover waste. The beginning of the bowel is the small intestine, sometimes referred to as the small bowel. This is where the useful nutrients are absorbed from what you eat. The small bowel delivers the waste to the colon, or large bowel. The colon is a 5-6 foot long muscular tube that delivers stool to the rectum. As the stool moves through the colon, the fluids are removed and absorbed into the body. The consistency of the stool is dependent upon many things, including how long the stool sits in the colon, how much of the water has been absorbed from the waste, and the amount of fiber and fluids in your diet. Stool consistency can vary from hard lumps to mushy to very loose, watery stool. The best and easiest consistency of stool is soft, like toothpaste; this consistency may be attained by adding fiber to your diet. Fiber helps move waste through the colon because it is indigestible by the human body. In other words, fiber adds ‘bulk’ to the stool. It is important to eat a diet high in fiber, however, most Americans lack fiber in their diet. -

Diagnosis and Management of Urinary Incontinence in Childhood
Committee 9 Diagnosis and Management of Urinary Incontinence in Childhood Chairman S. TEKGUL (Turkey) Members R. JM NIJMAN (The Netherlands), P. H OEBEKE (Belgium), D. CANNING (USA), W.BOWER (Hong-Kong), A. VON GONTARD (Germany) 701 CONTENTS E. NEUROGENIC DETRUSOR A. INTRODUCTION SPHINCTER DYSFUNCTION B. EVALUATION IN CHILDREN F. SURGICAL MANAGEMENT WHO WET C. NOCTURNAL ENURESIS G. PSYCHOLOGICAL ASPECTS OF URINARY INCONTINENCE AND ENURESIS IN CHILDREN D. DAY AND NIGHTTIME INCONTINENCE 702 Diagnosis and Management of Urinary Incontinence in Childhood S. TEKGUL, R. JM NIJMAN, P. HOEBEKE, D. CANNING, W.BOWER, A. VON GONTARD In newborns the bladder has been traditionally described as “uninhibited”, and it has been assumed A. INTRODUCTION that micturition occurs automatically by a simple spinal cord reflex, with little or no mediation by the higher neural centres. However, studies have indicated that In this chapter the diagnostic and treatment modalities even in full-term foetuses and newborns, micturition of urinary incontinence in childhood will be discussed. is modulated by higher centres and the previous notion In order to understand the pathophysiology of the that voiding is spontaneous and mediated by a simple most frequently encountered problems in children the spinal reflex is an oversimplification [3]. Foetal normal development of bladder and sphincter control micturition seems to be a behavioural state-dependent will be discussed. event: intrauterine micturition is not randomly distributed between sleep and arousal, but occurs The underlying pathophysiology will be outlined and almost exclusively while the foetus is awake [3]. the specific investigations for children will be discussed. For general information on epidemiology and During the last trimester the intra-uterine urine urodynamic investigations the respective chapters production is much higher than in the postnatal period are to be consulted.