AIS-Pennhip-Manual.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Thesis Has Been Submitted in Fulfilment of the Requirements for a Postgraduate Degree (E.G
This thesis has been submitted in fulfilment of the requirements for a postgraduate degree (e.g. PhD, MPhil, DClinPsychol) at the University of Edinburgh. Please note the following terms and conditions of use: This work is protected by copyright and other intellectual property rights, which are retained by the thesis author, unless otherwise stated. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given. ‘For the Good of the Breed’ Care, Ethics, and Responsibility in Pedigree Dog Breeding Chrissie Wanner PhD in Social Anthropology University of Edinburgh 2017 1 Declaration I declare that this thesis has been composed solely by myself and that it has not been submitted, in whole or in part, in any previous application for a degree. Except where states otherwise by reference or acknowledgment, the work presented is entirely my own. Signed: Date: 2 3 Abstract This thesis examines how the ethics of caring for pedigree dogs differ in the contexts of dog showing and veterinary practice. By highlighting conflicts around the shared use of ‘ordinary language’, I show how tensions between show‐world and veterinary perspectives relate to divergent understandings of ‘health’. Canine bodies speak to vets and breeders in conceptually different ways, so much so that breed‐specific features can be considered ‘perfect’ in the show‐ring yet ‘pathological’ in the veterinary clinic. -

Total Hip Replacement Part 1
DIAGNOSIS AND SURGICAL APPROACHES: TOTAL HIP REPLACEMENT PART 1 Jassin M. Jouria, MD Dr. Jassin M. Jouria is a medical doctor, professor of academic medicine, and medical author. He graduated from Ross University School of Medicine and has completed his clinical clerkship training in various teaching hospitals throughout New York, including King’s County Hospital Center and Brookdale Medical Center, among others. Dr. Jouria has passed all USMLE medical board exams, and has served as a test prep tutor and instructor for Kaplan. He has developed several medical courses and curricula for a variety of educational institutions. Dr. Jouria has also served on multiple levels in the academic field including faculty member and Department Chair. Dr. Jouria continues to serves as a Subject Matter Expert for several continuing education organizations covering multiple basic medical sciences. He has also developed several continuing medical education courses covering various topics in clinical medicine. Recently, Dr. Jouria has been contracted by the University of Miami/Jackson Memorial Hospital’s Department of Surgery to develop an e-module training series for trauma patient management. Dr. Jouria is currently authoring an academic textbook on Human Anatomy & Physiology. Abstract Arthritis, fractures, and repetitive strain can cause significant pain in the hip joint over time, but hip replacement surgery is an option for many patients each year in the United States. Plastic, ceramic, and metal components can be used to wholly replace the ball-and-socket hip joint and restore mobility in patients. Although most patients who undergo total hip replacement surgery are either retired or elderly, it can be useful for any patient who suffers pain that is not relieved by traditional methods. -

Inbreeding Purge of Canine Hip and Elbow Dysplasia
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 2 August 2020 doi:10.20944/preprints202008.0027.v1 Article Effects of Long Term Selection in the Border Collie Dog Breed: Inbreeding Purge of Canine Hip and Elbow Dysplasia Virág Ács1, György Kövér2, János Farkas2, Árpád Bokor3, István Nagy4 1Department of Animal Nutrition, Kaposvár University, Kaposvár, H-7400, 40, Guba S. str., Hungary; 2Department of Mathematics and Informatics, Kaposvár University, Kaposvár, H-7400, 40, Guba S. str., Hungary; 3Department of Hippology, Kaposvár University, Kaposvár, H-7400, 40, Guba S. str., Hungary; 4Department of Animal Science, Kaposvár University, Kaposvár, H-7400, 40, Guba S. str., Hungary; *Corresponding author: [email protected] Simple Summary: For dog breeders, health is one of the main criteria when choosing a breeding animal, thus selection for good anatomy is the key to reduce orthopedic disorders. In many dog breeds, radiographic screening for canine hip and elbow dysplasia is a compulsory test for breeding, however, these multifactorial traits are determined by genetic and environmental factors. Therefore, it is really hard to eliminate these disorders from the population. In natural selection, such traits can “purge” out of the with inbreeding. The study aimed to examine the inbreeding-purge of canine hip and elbow dysplasia in the border collie breed. The main conclusion was, that with over-representation of homozygous individuals may have a positive effect on hip and elbow conformation. Abstract: Pedigree data of 13 339 border collie dog was collected along with hip and elbow dysplasia records (1352 CHD and 524 CED), and an inbreeding-purging (IP) model was created to detect possible purging. -
Musculoskeletal Clinical Vignettes a Case Based Text
Leading the world to better health MUSCULOSKELETAL CLINICAL VIGNETTES A CASE BASED TEXT Department of Orthopaedic Surgery, RCSI Department of General Practice, RCSI Department of Rheumatology, Beaumont Hospital O’Byrne J, Downey R, Feeley R, Kelly M, Tiedt L, O’Byrne J, Murphy M, Stuart E, Kearns G. (2019) Musculoskeletal clinical vignettes: a case based text. Dublin, Ireland: RCSI. ISBN: 978-0-9926911-8-9 Image attribution: istock.com/mashuk CC Licence by NC-SA MUSCULOSKELETAL CLINICAL VIGNETTES Incorporating history, examination, investigations and management of commonly presenting musculoskeletal conditions 1131 Department of Orthopaedic Surgery, RCSI Prof. John O'Byrne Department of Orthopaedic Surgery, RCSI Dr. Richie Downey Prof. John O'Byrne Mr. Iain Feeley Dr. Richie Downey Dr. Martin Kelly Mr. Iain Feeley Dr. Lauren Tiedt Dr. Martin Kelly Department of General Practice, RCSI Dr. Lauren Tiedt Dr. Mark Murphy Department of General Practice, RCSI Dr Ellen Stuart Dr. Mark Murphy Department of Rheumatology, Beaumont Hospital Dr Ellen Stuart Dr Grainne Kearns Department of Rheumatology, Beaumont Hospital Dr Grainne Kearns 2 2 Department of Orthopaedic Surgery, RCSI Prof. John O'Byrne Department of Orthopaedic Surgery, RCSI Dr. Richie Downey TABLE OF CONTENTS Prof. John O'Byrne Mr. Iain Feeley Introduction ............................................................. 5 Dr. Richie Downey Dr. Martin Kelly General guidelines for musculoskeletal physical Mr. Iain Feeley examination of all joints .................................................. 6 Dr. Lauren Tiedt Dr. Martin Kelly Upper limb ............................................................. 10 Department of General Practice, RCSI Example of an upper limb joint examination ................. 11 Dr. Lauren Tiedt Shoulder osteoarthritis ................................................. 13 Dr. Mark Murphy Adhesive capsulitis (frozen shoulder) ............................ 16 Department of General Practice, RCSI Dr Ellen Stuart Shoulder rotator cuff pathology ................................... -

The Price of a Pedigree
The Price of a Pedigree DOG BREED STANDARDS AND BREED-RELATED ILLNESS The Price of a Pedigree: Dog breed standards and breed-related illness A report by Advocates for Animals 2006 Contents 1. Introduction: the welfare implications of pedigree dog breed standards 2. Current and future breeding trends 3. The prevalence of breed-related disease and abnormality 4. Breeds affected by hereditary hip and elbow dysplasia 4.1 The British Veterinary Association/Kennel Club hip and elbow dysplasia schemes 4.2 International studies of the prevalence of hip and elbow dysplasia 5. Breeds affected by inherited eye diseases 5.1 The British Veterinary Association/Kennel Club/ISDS Eye scheme 5.2 Further breed-related eye problems 6. Breeds affected by heart and respiratory disease 6.1 Brachycephalic Upper Airway Syndrome 6.2 Increased risk of heart conditions 7. Breed-related skin diseases 8. Inherited skeletal problems of small and long-backed breeds 8.1 Luxating patella 8.2 Intervertebral disc disease in chondrodystrophoid breeds 9. Bone tumours in large and giant dog breeds 10. Hereditary deafness 11. The Council of Europe and breed standards 11.1 Views of companion animal organisations on dog breeding 12. Conclusions and recommendations Appendix. Scientific assessments of the prevalence of breed-related disorders in pedigree dogs. Tables 1 – 9 and Glossaries of diseases References 1. Introduction: The welfare implications of pedigree dog breed standards ‘BREEDERS AND SCIENTISTS HAVE LONG BEEN AWARE THAT ALL IS NOT WELL IN THE WORLD OF COMPANION ANIMAL BREEDING.’ Animal Welfare, vol 8, 1999 1 There were an estimated 6.5 million dogs in the UK in 2003 and one in five of all households includes a dog.2 Only a minority (around a quarter) of these dogs are mongrels or mixed breed dogs. -

Total Hip Arthroplasty in the Horse: Overview, Technical Considerations and Case Report N
EQUINE VETERINARY EDUCATION / AE / November 2010 547 Case Reporteve_118 547..553 Total hip arthroplasty in the horse: Overview, technical considerations and case report N. Huggons*, R. Andrea†, B. Grant‡ and C. Duncan§ WR Pritchard Veterinary Medical Teaching Hospital, University of California Davis; †Chaparral Veterinary Medical Center, Arizona; ‡31624 Wrightwood, California, USA; §Gordon & Leslie Diamond Health Care Centre, Vancouver, Canada. Keywords: horse; total hip arthroplasty; hip replacement; coxofemoral joint; acetabulum Summary Hance 1998; Loesch et al. 2003; Smith et al. 2004), disruption of intra-articular ligaments (Trotter et al. 1986; A total hip arthroplasty was performed in a small Nixon 1994), fractures (Turner et al. 1979; Embertson equine patient with a history of traumatic subluxation of et al. 1986; Hunt et al. 1990a) and dysplasia (Jogi and the coxofemoral joint during infancy resulting in severe Norberg 1962; Speirs and Wrigley 1979) have also been degenerative changes to the femoral head and described. acetabulum. The transtrochanteric surgical approach Few treatment options exist for hip disease, with used to expose the joint, as well as the technique and limitations primarily due to the large muscle mass technology to replace the joint, is described. The patient covering the joint, difficulty with surgical access to the was weightbearing within 24 h of surgery and walking joint and implant failure associated with post operative successfully without sling support 4 days post operatively. weightbearing. Surgical fixation of femoral capital On the fifth post operative day, the patient abruptly epiphyseal fractures in foals has included excision deteriorated and succumbed to multiple pulmonary arthroplasty, Steinmann or Knowles pin fixation, multiple thromboemboli and a jejunal infarction. -
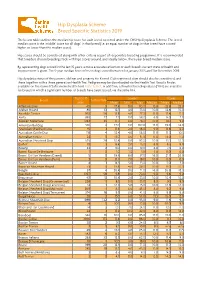
Hip Dysplasia Scheme Breed Specific Statistics 2019
Hip Dysplasia Scheme Breed Specific Statistics 2019 The below table outlines the median hip score for each breed screened under the CHS Hip Dysplasia Scheme. The breed median score is the ‘middle’ score for all dogs’ in that breed (i.e. an equal number of dogs in that breed have scored higher or lower than the median score). Hip scores should be considered along with other criteria as part of responsible breeding programme. It is recommended that breeders choose breeding stock with hips scores around, and ideally below, the 5-year breed median score. By representing dogs scored in the last 15 years, a more accurate reflection of each breed’s current state of health and improvement is given. The 5-year median here refers to dogs scored between 1st January 2015 and 31st December 2019. Hip dysplasia status of the parents, siblings and progeny for Kennel Club registered dogs should also be considered, and these together with a three generation Health Test Pedigree may be downloaded via the Health Test Results Finder, available on the Kennel Club's online health tool Mate Select. In addition, estimated breeding values (EBVs) are available for breeds in which a significant number of breeds have been scored, via the same link. Tested 15 15 years 5 years Breed Tested 2019 years Mean Min Max Median Mean Median Affenpinscher 40 0 17.9 8.0 90.0 13.0 23.8 23.0 Afghan Hound 85 33 12.3 4.0 73.0 10.0 12.6 10.0 Airedale Terrier 910 58 13.9 4.0 77.0 11.0 13.8 11.0 Akita 883 27 7.7 0.0 58.0 6.0 8.0 7.0 Alaskan Malamute 1242 25 11.7 0.0 78.0 10.0 10.1 9.0 -

The Orthopedic Examination in Dogs: a Fast and Effective Tool Denis Marcellin-Little, DEDV, DACVS, DACVSMR University of California, Davis
The Orthopedic Examination in Dogs: A Fast and Effective Tool Denis Marcellin-Little, DEDV, DACVS, DACVSMR University of California, Davis Even if many lamenesses in dogs appear somewhat mysterious at first glance, they can be approached step by step, and their cause can, in most instances, be localized to specific bones or joints. The purpose of this presentation is to describe these diagnostic steps: know the most common orthopedic disorders, keep the signalment in mind, collect a thorough history, observe the dog standing, changing position, and walking, palpate the dog, prepare a short list of differential diagnoses, and confirm your suspicions with radiographs. STEP 1. KNOW THE MOST COMMON ORTHOPEDIC DISORDERS The majority of lamenesses are caused by a handful of diseases.9 In the forelimb, elbow dysplasia with or without fragmentation of the medial coronoid process and osteochondritis dissecans of the humeral head are most common. In the hind limb, hip dysplasia and cranial cruciate ligament injuries are most common (Table 1). Table 1. Orthopedic problems ranked by decreasing prevalence Problem Incidence Canine hip dysplasia 10% Pelvic fracture 8% Cruciate ligament injuries 8% Patellar luxation 7% Femoral fractures 7% Coxofemoral luxation 5% Radial and ulnar fractures 4% Other 51% If we extend the list to 20 diseases, we cover 99 percent of the lamenesses. Ideally, we should have in mind one or two statements about the 1) definition, 2) importance (prevalence and severity), 3) etiology, 4) pathogenesis, 5) heritability, 6) clinical signs, 7) diagnosis, 8) treatment, and 9) prognosis of each common orthopedic problem. For example, Table 2 lists the facts related to canine hip dysplasia. -

Uživatel:Zef/Output18
Uživatel:Zef/output18 < Uživatel:Zef rozřadit, rozdělit na více článků/poznávaček; Název !! Klinický obraz !! Choroba !! Autor Bárányho manévr; Bonnetův manévr; Brudzinského manévr; Fournierův manévr; Fromentův manévr; Heimlichův manévr; Jendrassikův manévr; Kernigův manévr; Lasčgueův manévr; Müllerův manévr; Scanzoniho manévr; Schoberův manévr; Stiborův manévr; Thomayerův manévr; Valsalvův manévr; Beckwithova známka; Sehrtova známka; Simonova známka; Svěšnikovova známka; Wydlerova známka; Antonovo znamení; Apleyovo znamení; Battleho znamení; Blumbergovo znamení; Böhlerovo znamení; Courvoisierovo znamení; Cullenovo znamení; Danceovo znamení; Delbetovo znamení; Ewartovo znamení; Forchheimerovo znamení; Gaussovo znamení; Goodellovo znamení; Grey-Turnerovo znamení; Griesingerovo znamení; Guddenovo znamení; Guistovo znamení; Gunnovo znamení; Hertogheovo znamení; Homansovo znamení; Kehrerovo znamení; Leserovo-Trélatovo znamení; Loewenbergerovo znamení; Minorovo znamení; Murphyho znamení; Nobleovo znamení; Payrovo znamení; Pembertonovo znamení; Pinsovo znamení; Pleniesovo znamení; Pléniesovo znamení; Prehnovo znamení; Rovsingovo znamení; Salusovo znamení; Sicardovo znamení; Stellwagovo znamení; Thomayerovo znamení; Wahlovo znamení; Wegnerovo znamení; Zohlenovo znamení; Brachtův hmat; Credého hmat; Dessaignes ; Esmarchův hmat; Fritschův hmat; Hamiltonův hmat; Hippokratův hmat; Kristellerův hmat; Leopoldovy hmat; Lepagův hmat; Pawlikovovy hmat; Riebemontův-; Zangmeisterův hmat; Leopoldovy hmaty; Pawlikovovy hmaty; Hamiltonův znak; Spaldingův znak; -

Registration Regulations Empowerment
New Zealand Kennel Club (Inc) (Affiliated with The Kennel Club, England) (Associated with the Federation Cynologique Internationale) REGISTRATION REGULATIONS (Reprinted with Additions and Amendments, to 1 April 2021) Headquarters Prosser Street, Porirua. New Zealand Kennel Club Private Bag 50903 Porirua 5240 Copyright - New Zealand Kennel Club (Inc.) Page | 1 CONTENTS Principles of the Registration System Section I - Registration Regulations Empowerment ........................................................................................................................ 4 Definitions .............................................................................................................................. 4 Charges, Fees, Forms and Signatures. ................................................................................. 4 The Executive Council, Registry and the Administration ........................................................ 5 The Register .......................................................................................................................... 5 Executive Council Powers ...................................................................................................... 6 Registry Details ...................................................................................................................... 6 Litter Notification .................................................................................................................... 6 Registration of Dogs. ............................................................................................................ -

Congenital Hip Dysplasia Screening
Newborn Critical Care Center (NCCC) Guidelines Developmental Dysplasia of the Hip BACKGROUND Developmental dysplasia of the hip (DDH) includes a range of hip abnormalities in which the femoral head and acetabulum are improperly aligned (i.e., dislocated, dislocatable or subluxated), leading to abnormal growth. DDH can lead to lifetime morbidity including gait abnormalities, pain and degenerative arthritis. SCREENING The goal of screening is to prevent a subluxated or dislocated hip by 6 to 12 months of age. The physical examination is the most important component of a DDH screening program, with imaging playing a secondary role. As such, all infants admitted to the NCCC should be screened for DDH by clinical hip exam. Clinical Exam The Ortolani maneuver is the most important clinical test for detecting newborn hip dysplasia. To perform the Ortolani test, gently lift the flexed thigh and push the greater trochanter anteriorly (reducing dislocation). A positive sign is a distinctive 'clunk' which can be heard and felt as the femoral head relocates anteriorly into the acetabulum. (Of note, the Barlow maneuver, in which the femoral head is adducted until it becomes subluxated or dislocated, has no proven predictive value for future hip dislocation. If it is performed, care should be taken to avoid any posterior-directed force during adduction, as it is possible that the maneuver itself could create hip instability.) At a minimum, clinical hip exams for infants in the NCCC should be performed shortly after birth and at the time of discharge. Additional, periodic exams may be performed according to clinical discretion. In addition to periodic Ortolani tests, infants should be observed for limited or asymmetric hip abduction after the neonatal period. -

A Retrospective Study on Findings of Canine Hip Dysplasia Screening in Kenya
Veterinary World, EISSN: 2231-0916 RESEARCH ARTICLE Available at www.veterinaryworld.org/Vol.8/November-2015/10.pdf Open Access A retrospective study on findings of canine hip dysplasia screening in Kenya Peter Kimeli1, Susan W. Mbugua1, Roger M. Cap2, Gilbert Kirui1, Tequiero O. Abuom1, Willy E. Mwangi1, Ambrose N. Kipyegon1 and John D. Mande1 1. Department of Clinical Studies, Faculty of Veterinary Medicine, University of Nairobi, P.O. Box 29053-00625, Kangemi, Kenya; 2. Sercombe Veterinary Surgeons, P.O Box 24878-00502, Nairobi, Kenya. Corresponding author: Peter Kimeli, e-mail: [email protected], SWM: [email protected], RMC: [email protected], GK: [email protected], TOA: [email protected], WEM: [email protected], ANK: [email protected], JDM: [email protected] Received: 15-06-2015, Revised: 27-09-2015, Accepted: 14-10-2015, Published online: 22-11-2015 doi: 10.14202/vetworld.2015.1326-1330 How to cite this article: Kimeli P, Mbugua SW, Cap RM, Kirui G, Abuom TO, Mwangi WE, Kipyegon AN, Mande JD (2015) A retrospective study on findings of canine hip dysplasia screening in Kenya, Veterinary World 8(11): 1326-1330. Abstract Aim: The current study was undertaken to evaluate the findings of canine hip dysplasia screening in Kenya. Materials and Methods: Records for 591 dogs were included in this study. The data was obtained from the national screening office, Kenya Veterinary Board, for the period between the years 1998 and 2014. Monthly screening records were assessed and information relating to year of evaluation, breed, sex, age, and hip score captured. Descriptive statistics of hip scores was computed based on year, sex, age, and breed.