Chapter 20 Magnetic Properties
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
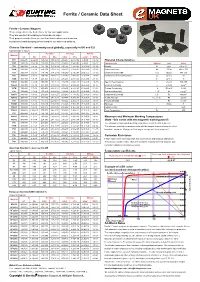
Ferrite / Ceramic Data Sheet
Ferrite / Ceramic Data Sheet Ferrite / Ceramic Magnets These magnets are the best choice for low cost applications. They are excellent at resisting corrosion due to water. Their properties make them an excellent choice when used in motors, loudspeakers and clamping devices and for use with reed switches. Chinese Standard - commonly used globally, especially in UK and EU Typical Range of Values Br Hc (Hcb) Hci (Hcj) BHmax Material mT kG kA/m kOe kA/m kOe kJ/m3 MGOe Y8T 200-235 2.0-2.35 125-160 1.57-2.01 210-280 2.64-3.52 6.5-9.5 0.8-1.2 Physical Characteristics Y10T 200-235 2.0-2.35 128-160 1.61-2.01 210-280 2.64-3.52 6.4-9.6 0.8-1.2 Characteristic Symbol Unit Value Y20 320-380 3.2-3.8 135-190 1.70-2.39 140-195 1.76-2.45 18.0-22.0 2.3-2.8 Density D g/cc 4.9 to 5.1 Y22H 310-360 3.1-3.6 220-250 2.76-3.14 280-320 3.52-4.02 20.0-24.0 2.5-3.0 Vickers Hardness Hv D.P.N 400 to 700 Y23 320-370 3.2-3.7 170-190 2.14-2.39 190-230 2.39-2.89 20.0-25.5 2.5-3.2 Compression Strength C.S N/mm2 680-720 Y25 360-400 3.6-4.0 135-170 1.70-2.14 140-200 1.76-2.51 22.5-28.0 2.8-3.5 Coefficient of Thermal Expansion C// 10-6/C 15 Y26H 360-390 3.6-3.9 220-250 2.76-3.14 225-255 2.83-3.20 23.0-28.0 2.9-3.5 C^ 10-6/C 10 Y26H-1 360-390 3.6-3.9 200-250 2.51-3.14 225-255 2.83-3.20 23.0-28.0 2.9-3.5 Specific Heat Capacity c J/kg°C 795-855 Y26H-2 360-380 3.6-3.8 263-288 3.30-3.62 318-350 4.00-4.40 24.0-28.0 3.0-3.5 Electrical Resistivity r m Ω.cm 1x1010 Y27H 370-400 3.7-4.0 205-250 2.58-3.14 210-255 2.64-3.20 25.0-29.0 3.1-3.6 Thermal Conductivity k W/cm°C 0.029 Y28 370-400 3.7-4.0 175-210 2.20-2.64 180-220 2.26-2.76 26.0-30.0 3.3-3.8 Modulus of Elasticity l / E Pa 1.8x1011 Y28H-1 380-400 3.8-4.0 240-260 3.02-3.27 250-280 3.14-3.52 27.0-30.0 3.4-3.8 Compression Strength C.S. -

Canted Ferrimagnetism and Giant Coercivity in the Non-Stoichiometric
Canted ferrimagnetism and giant coercivity in the non-stoichiometric double perovskite La2Ni1.19Os0.81O6 Hai L. Feng1, Manfred Reehuis2, Peter Adler1, Zhiwei Hu1, Michael Nicklas1, Andreas Hoser2, Shih-Chang Weng3, Claudia Felser1, Martin Jansen1 1Max Planck Institute for Chemical Physics of Solids, Dresden, D-01187, Germany 2Helmholtz-Zentrum Berlin für Materialien und Energie, Berlin, D-14109, Germany 3National Synchrotron Radiation Research Center (NSRRC), Hsinchu, 30076, Taiwan Abstract: The non-stoichiometric double perovskite oxide La2Ni1.19Os0.81O6 was synthesized by solid state reaction and its crystal and magnetic structures were investigated by powder x-ray and neutron diffraction. La2Ni1.19Os0.81O6 crystallizes in the monoclinic double perovskite structure (general formula A2BB’O6) with space group P21/n, where the B site is fully occupied by Ni and the B’ site by 19 % Ni and 81 % Os atoms. Using x-ray absorption spectroscopy an Os4.5+ oxidation state was established, suggesting presence of about 50 % 5+ 3 4+ 4 paramagnetic Os (5d , S = 3/2) and 50 % non-magnetic Os (5d , Jeff = 0) ions at the B’ sites. Magnetization and neutron diffraction measurements on La2Ni1.19Os0.81O6 provide evidence for a ferrimagnetic transition at 125 K. The analysis of the neutron data suggests a canted ferrimagnetic spin structure with collinear Ni2+ spin chains extending along the c axis but a non-collinear spin alignment within the ab plane. The magnetization curve of La2Ni1.19Os0.81O6 features a hysteresis with a very high coercive field, HC = 41 kOe, at T = 5 K, which is explained in terms of large magnetocrystalline anisotropy due to the presence of Os ions together with atomic disorder. -

Basic Magnetic Measurement Methods
Basic magnetic measurement methods Magnetic measurements in nanoelectronics 1. Vibrating sample magnetometry and related methods 2. Magnetooptical methods 3. Other methods Introduction Magnetization is a quantity of interest in many measurements involving spintronic materials ● Biot-Savart law (1820) (Jean-Baptiste Biot (1774-1862), Félix Savart (1791-1841)) Magnetic field (the proper name is magnetic flux density [1]*) of a current carrying piece of conductor is given by: μ 0 I dl̂ ×⃗r − − ⃗ 7 1 - vacuum permeability d B= μ 0=4 π10 Hm 4 π ∣⃗r∣3 ● The unit of the magnetic flux density, Tesla (1 T=1 Wb/m2), as a derive unit of Si must be based on some measurement (force, magnetic resonance) *the alternative name is magnetic induction Introduction Magnetization is a quantity of interest in many measurements involving spintronic materials ● Biot-Savart law (1820) (Jean-Baptiste Biot (1774-1862), Félix Savart (1791-1841)) Magnetic field (the proper name is magnetic flux density [1]*) of a current carrying piece of conductor is given by: μ 0 I dl̂ ×⃗r − − ⃗ 7 1 - vacuum permeability d B= μ 0=4 π10 Hm 4 π ∣⃗r∣3 ● The Physikalisch-Technische Bundesanstalt (German national metrology institute) maintains a unit Tesla in form of coils with coil constant k (ratio of the magnetic flux density to the coil current) determined based on NMR measurements graphics from: http://www.ptb.de/cms/fileadmin/internet/fachabteilungen/abteilung_2/2.5_halbleiterphysik_und_magnetismus/2.51/realization.pdf *the alternative name is magnetic induction Introduction It -

Electrostatics Vs Magnetostatics Electrostatics Magnetostatics
Electrostatics vs Magnetostatics Electrostatics Magnetostatics Stationary charges ⇒ Constant Electric Field Steady currents ⇒ Constant Magnetic Field Coulomb’s Law Biot-Savart’s Law 1 ̂ ̂ 4 4 (Inverse Square Law) (Inverse Square Law) Electric field is the negative gradient of the Magnetic field is the curl of magnetic vector electric scalar potential. potential. 1 ′ ′ ′ ′ 4 |′| 4 |′| Electric Scalar Potential Magnetic Vector Potential Three Poisson’s equations for solving Poisson’s equation for solving electric scalar magnetic vector potential potential. Discrete 2 Physical Dipole ′′′ Continuous Magnetic Dipole Moment Electric Dipole Moment 1 1 1 3 ∙̂̂ 3 ∙̂̂ 4 4 Electric field cause by an electric dipole Magnetic field cause by a magnetic dipole Torque on an electric dipole Torque on a magnetic dipole ∙ ∙ Electric force on an electric dipole Magnetic force on a magnetic dipole ∙ ∙ Electric Potential Energy Magnetic Potential Energy of an electric dipole of a magnetic dipole Electric Dipole Moment per unit volume Magnetic Dipole Moment per unit volume (Polarisation) (Magnetisation) ∙ Volume Bound Charge Density Volume Bound Current Density ∙ Surface Bound Charge Density Surface Bound Current Density Volume Charge Density Volume Current Density Net , Free , Bound Net , Free , Bound Volume Charge Volume Current Net , Free , Bound Net ,Free , Bound 1 = Electric field = Magnetic field = Electric Displacement = Auxiliary -

Magnetism, Magnetic Properties, Magnetochemistry
Magnetism, Magnetic Properties, Magnetochemistry 1 Magnetism All matter is electronic Positive/negative charges - bound by Coulombic forces Result of electric field E between charges, electric dipole Electric and magnetic fields = the electromagnetic interaction (Oersted, Maxwell) Electric field = electric +/ charges, electric dipole Magnetic field ??No source?? No magnetic charges, N-S No magnetic monopole Magnetic field = motion of electric charges (electric current, atomic motions) Magnetic dipole – magnetic moment = i A [A m2] 2 Electromagnetic Fields 3 Magnetism Magnetic field = motion of electric charges • Macro - electric current • Micro - spin + orbital momentum Ampère 1822 Poisson model Magnetic dipole – magnetic (dipole) moment [A m2] i A 4 Ampere model Magnetism Microscopic explanation of source of magnetism = Fundamental quantum magnets Unpaired electrons = spins (Bohr 1913) Atomic building blocks (protons, neutrons and electrons = fermions) possess an intrinsic magnetic moment Relativistic quantum theory (P. Dirac 1928) SPIN (quantum property ~ rotation of charged particles) Spin (½ for all fermions) gives rise to a magnetic moment 5 Atomic Motions of Electric Charges The origins for the magnetic moment of a free atom Motions of Electric Charges: 1) The spins of the electrons S. Unpaired spins give a paramagnetic contribution. Paired spins give a diamagnetic contribution. 2) The orbital angular momentum L of the electrons about the nucleus, degenerate orbitals, paramagnetic contribution. The change in the orbital moment -

Stress Dependence of the Magnetic Properties of Steels Michael K
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1992 Stress dependence of the magnetic properties of steels Michael K. Devine Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Metallurgy Commons Recommended Citation Devine, Michael K., "Stress dependence of the magnetic properties of steels" (1992). Retrospective Theses and Dissertations. 227. https://lib.dr.iastate.edu/rtd/227 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Stress dependence of the magnetic properties of steels by Michael Kenneth Devine A Thesis Submitted to the Graduate Faculty in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Department: Materials Science and Engineering Major: Metallurgy Approved: Signature redacted for privacy In Charge of Major Work For the Major Department For the Graduate College Iowa State University Ames, Iowa 1992 ii TABLE OF CONTENTS Page CHAPTER 1. INTRODUCTION • • • • • • 0 . • • 1 Origin of Ferromagnetism • . 3 Influence of Microstructure and Composition 5 Influence of Stress . • • • 0 • • 11 NDE Applications • • 28 Statement of Problem and Experimental Approach • 29 CHAPTER 2. EXPERIMENTAL PROCEDURE FOR MAGNETIC MEASUREMENTS • • • • • • • • ~0 Magnescope Instrumentation 30 Inspection Head Design • • • 32 CHAPTER 3. LABORATORY SCALE STRESS DETECTION WITH A SOLENOID • • • • • • • • • • • • • • • • • • 37 Introduction • • . 37 Materials and Experimental Procedure 37 Results and Discussion • • 38 Conclusions • • • • • • • • • • • • • • • • 0 • • • 45 CHAPTER 4. -

A TWO DIMENSIONAL FERRITE-CORE MEMORY Bv M
A TWO DIMENSIONAL FERRITE-CORE MEMORY Bv M. M. FAROOQUI, S. P. SKiVASTAVA AND R. N. NEOGI (Tata Institute of Fundamental Research, Bomba),) Received January t0, 1957 (Communicated by Prof. Bernard P ABS'IXACT This paper describes a two dimensional matrix memory using ferrite- cores. A urtit consisting of 100 words of I 1 binary digits each has been cortstructed for parallel operation. The word drive is from a biasr switch-core matrix, while the digit drive makes use of pulse--trans- formers. Logic and circuit techniques enable high discrimination bet- ween wanted and unwanted signals. A semi-automatic method for testing the memory cores is also described. INTRODUCTION THE potentiality of magnetie fer¡ with a rectangular hysteresis loop as binary storage elements for digital computer was realised earlier by Forrester. x Later, Papian% s and almost simultaneously Rajchman4, ~ showed the practicability of such a system by successfully operating memories of fairly large capacity. This paper describes a modest attempt in this direction. A memory of I00 words, 11 bits each, has beca constructed to operate with a smaU digital computer at the Tata institute of Fundamental Research, Bombay. THE PRINClPLE OF OPERATION OF THE MEMORY The two stable states required for sto¡ binary information in magnetic cores ate the states of positive and negative magnetisation. These are the states corresponding to the position A0 and Ax on the hysteresis loop and can be termed as the 0- and the 1-states respectively (Fig. 2). Con- sider a number of sucia cores with ah ideaUy rectangular hysteresis character- istic, arranged in rows and columns in the form of a matrix. -

Selecting the Optimal Inductor for Power Converter Applications
Selecting the Optimal Inductor for Power Converter Applications BACKGROUND SDR Series Power Inductors Today’s electronic devices have become increasingly power hungry and are operating at SMD Non-shielded higher switching frequencies, starving for speed and shrinking in size as never before. Inductors are a fundamental element in the voltage regulator topology, and virtually every circuit that regulates power in automobiles, industrial and consumer electronics, SRN Series Power Inductors and DC-DC converters requires an inductor. Conventional inductor technology has SMD Semi-shielded been falling behind in meeting the high performance demand of these advanced electronic devices. As a result, Bourns has developed several inductor models with rated DC current up to 60 A to meet the challenges of the market. SRP Series Power Inductors SMD High Current, Shielded Especially given the myriad of choices for inductors currently available, properly selecting an inductor for a power converter is not always a simple task for designers of next-generation applications. Beginning with the basic physics behind inductor SRR Series Power Inductors operations, a designer must determine the ideal inductor based on radiation, current SMD Shielded rating, core material, core loss, temperature, and saturation current. This white paper will outline these considerations and provide examples that illustrate the role SRU Series Power Inductors each of these factors plays in choosing the best inductor for a circuit. The paper also SMD Shielded will describe the options available for various applications with special emphasis on new cutting edge inductor product trends from Bourns that offer advantages in performance, size, and ease of design modification. -

Chapter 6 Antiferromagnetism and Other Magnetic Ordeer
Chapter 6 Antiferromagnetism and Other Magnetic Ordeer 6.1 Mean Field Theory of Antiferromagnetism 6.2 Ferrimagnets 6.3 Frustration 6.4 Amorphous Magnets 6.5 Spin Glasses 6.6 Magnetic Model Compounds TCD February 2007 1 1 Molecular Field Theory of Antiferromagnetism 2 equal and oppositely-directed magnetic sublattices 2 Weiss coefficients to represent inter- and intra-sublattice interactions. HAi = n’WMA + nWMB +H HBi = nWMA + n’WMB +H Magnetization of each sublattice is represented by a Brillouin function, and each falls to zero at the critical temperature TN (Néel temperature) Sublattice magnetisation Sublattice magnetisation for antiferromagnet TCD February 2007 2 Above TN The condition for the appearance of spontaneous sublattice magnetization is that these equations have a nonzero solution in zero applied field Curie Weiss ! C = 2C’, P = C’(n’W + nW) TCD February 2007 3 The antiferromagnetic axis along which the sublattice magnetizations lie is determined by magnetocrystalline anisotropy Response below TN depends on the direction of H relative to this axis. No shape anisotropy (no demagnetizing field) TCD February 2007 4 Spin Flop Occurs at Hsf when energies of paralell and perpendicular configurations are equal: HK is the effective anisotropy field i 1/2 This reduces to Hsf = 2(HKH ) for T << TN Spin Waves General: " n h q ~ q ! M and specific heat ~ Tq/n Antiferromagnet: " h q ~ q ! M and specific heat ~ Tq TCD February 2007 5 2 Ferrimagnetism Antiferromagnet with 2 unequal sublattices ! YIG (Y3Fe5O12) Iron occupies 2 crystallographic sites one octahedral (16a) & one tetrahedral (24d) with O ! Magnetite(Fe3O4) Iron again occupies 2 crystallographic sites one tetrahedral (8a – A site) & one octahedral (16d – B site) 3 Weiss Coefficients to account for inter- and intra-sublattice interaction TCD February 2007 6 Below TN, magnetisation of each sublattice is zero. -

Magnetics in Switched-Mode Power Supplies Agenda
Magnetics in Switched-Mode Power Supplies Agenda • Block Diagram of a Typical AC-DC Power Supply • Key Magnetic Elements in a Power Supply • Review of Magnetic Concepts • Magnetic Materials • Inductors and Transformers 2 Block Diagram of an AC-DC Power Supply Input AC Rectifier PFC Input Filter Power Trans- Output DC Outputs Stage former Circuits (to loads) 3 Functional Block Diagram Input Filter Rectifier PFC L + Bus G PFC Control + Bus N Return Power StageXfmr Output Circuits + 12 V, 3 A - + Bus + 5 V, 10 A - PWM Control + 3.3 V, 5 A + Bus Return - Mag Amp Reset 4 Transformer Xfmr CR2 L3a + C5 12 V, 3 A CR3 - CR4 L3b + Bus + C6 5 V, 10 A CR5 Q2 - + Bus Return • In forward converters, as in most topologies, the transformer simply transmits energy from primary to secondary, with no intent of energy storage. • Core area must support the flux, and window area must accommodate the current. => Area product. 4 3 ⎛ PO ⎞ 4 AP = Aw Ae = ⎜ ⎟ cm ⎝ K ⋅ΔB ⋅ f ⎠ 5 Output Circuits • Popular configuration for these CR2 L3a voltages---two secondaries, with + From 12 V 12 V, 3 A a lower voltage output derived secondary CR3 C5 - from the 5 V output using a mag CR4 L3b + amp postregulator. From 5 V 5 V, 10 A secondary CR5 C6 - CR6 L4 SR1 + 3.3 V, 5 A CR8 CR7 C7 - Mag Amp Reset • Feedback to primary PWM is usually from the 5 V output, leaving the +12 V output quasi-regulated. 6 Transformer (cont’d) • Note the polarity dots. Xfmr CR2 L3a – Outputs conduct while Q2 is on. -

Module 3 : MAGNETIC FIELD Lecture 20 : Magnetism in Matter
Module 3 : MAGNETIC FIELD Lecture 20 : Magnetism in Matter Objectives In this lecture you will learn the following Study magnetic properties of matter. Express Ampere's law in the presence of magnetic matter. Define magnetization and H-vector. Understand displacement current. Assemble all the Maxwell's equations together. Study properties and propagation of electromagnetic waves in vacuum. Magnetism in Matter In our discussion on electrostatics, we have seen that in the presence of an electric field, a dielectric gets polarized, leading to bound charges. The polarization vector is, in general, in the direction of the applied electric field. A similar phenomenon occurs when a material medium is subjected to an external magnetic field. However, unlike the behaviour of dielectrics in electric field, different types of material behave in different ways when an external magnetic field is applied. We have seen that the source of magnetic field is electric current. The circulating electrons in an atom, being tiny current loops, constitute a magnetic dipole with a magnetic moment whose direction depends on the direction in which the electron is moving. An atom as a whole, may or may not have a net magnetic moment depending on the way the moments due to different elecronic orbits add up. (The situation gets further complicated because of electron spin, which is a purely quantum concept, that provides an intrinsic magnetic moment to an electron.) In the absence of a magnetic field, the atomic moments in a material are randomly oriented and consequently the net magnetic moment of the material is zero. However, in the presence of a magnetic field, the substance may acquire a net magnetic moment either in the direction of the applied field or in a direction opposite to it. -

High Coercivity Vs
Card Testing International Ltd Level 4, 105 High Street PO Box 30 356 Lower Hutt 5040, NZ Phone: Asia/Pacific +64 4 903 4990 North America (800) 438 9036 Email: [email protected] Website: www.cardtest.com High Coercivity vs. Low Coercivity Accidental magnetic card erasure, a problem today? We have come to expect reliability in our electronic system. Yes, repairs are required, but when you add up all the electronic gadgets in our homes and businesses it's a miracle that service isn't needed more often. Banking, vending, identification and security are some of the most important systems in our lives, and it is critical that they work smoothly. Some of the cards we use in these systems are subject to reliability problems depending on the material used in their tape. The standard magnetic tape that is used on many bank cards are made with iron oxide, effectively rust. The magnetism needed to erase this medium is called coercive force. Units of coercivity are named Oersted. The coercivity of the iron oxide tape used in banking and many other application is 300 Oersted. Ordinary magnets with which we come into contact can easily erase this tape. The magnets that are sometimes used to hold messages onto our refrigerators fall into this category. Some handbags/wallets have magnetic latches that can be a problem. Magnetic force falls off rapidly as the card distance from the offending magnet is increased. It's unlikely that an ordinary magnet kept at least 10 to 15mm from the stripe will damage the tape.