Identifying Invertebrate Representation Within Fiji’S Future Network of Forest Reserves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Curriculum Vitae – 2018 1
Steven W. Lingafelter, Ph. D. PRESENT POSITION (2016-PRESENT) Entomologist-Identifier, APHIS-PPQ-USDA, Douglas, Arizona, 85607 Phone: (office) 520-805-4458; (cell) 520-368-8426 PREVIOUS POSITION (1996-2016) Research Entomologist, Systematic Entomology Laboratory, USDA, National Museum of Natural History, Washington, DC. SCIENTIFIC PHILOSOPHY To build a global understanding of biological diversity by exploiting all tools for discovery, description, and dissemination EDUCATION 1996. Ph.D. Entomology. University of Kansas, J. S. Ashe, advisor 1991. M.S. Biology. Midwestern State University, N. V. Horner, advisor 1989. B.S. Biology. Midwestern State University, N. V. Horner, advisor APPOINTMENTS AND PROFESSIONAL SERVICE 2016–present. Entomologist-Identifier, USDA-APHIS-PPQ 2016–present. University of Arizona Insect Collection Associate 2010–2016. Coleoptera Lead Scientist, USDA-Agriculture Research Service 2014–2016. Advisor to ORISE-DoE Post-Doc consultant on beetle identifications 2011–2014. Project Manager and Advisor to USDA Post-Doc for project on the Emerald Ash Borer 2006–2011. Advisor to USDA-APHIS-PPQ Post-Doc for weevil identifications 2012–2015. Co-Editor Proceedings of Entomological Society of Washington 2011–2015. Subject Editor ZooKeys 2010–2016. Editorial Board Entomological Research, Korea 2005–2009. Managing Editor Coleopterists Bulletin 2005–2006. President Entomological Society of Washington 2004–2005. Adjunct Associate Professor University of Florida, Gainesville 2000–2006. Adjunct Associate Professor University of Maryland, College Park 1996–2016. Adjunct Scientist Smithsonian Institution Lingafelter – Curriculum vitae – 2018 1 PEER REVIEWED BOOKS & MAJOR MONOGRAPHS (OVER 100 PAGES, CHRONOLOGICAL LISTING) 1998. Lingafelter, S. W. The genera of Elaphidiini Thomson (Coleoptera: Cerambycidae). Memoirs of the Washington Entomological Society, No. 20. 118 pp. -
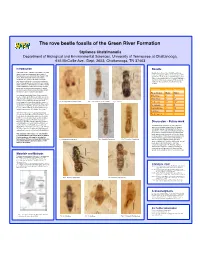
The Rove Beetle Fossils of the Green River Formation
The rove beetle fossils of the Green River Formation! Stylianos Chatzimanolis" Department of Biological and Environmental Sciences, University of Tennessee at Chattanooga, " 615 McCallie Ave., Dept. 2653, Chattanooga, TN 37403! ! Introduction! Results! ! The Green River Formation is a system of Eocene Examination of more than 10,000 fossiliferous lakes located near the present day borders of rocks over a period of three weeks produced 354 Wyoming, Colorado and Utah. Over the past 150 specimens of rove beetles. A preliminary account years, there have been several astonishing of those revealed the presence of eight subfamilies vertebrate discoveries from these fossil beds, and at least 25 new species (see Table below) in including crocodiles, boa constrictors, numerous addition to the specimens described before by fish and the earliest known bat (Nuds and Selden Scudder.! 2008). The flora of the area has also been studied in detail (MacGinitie 1969) showing that cut-tails, ! palms and sycamores were common. However, the insect fauna remains relatively neglected with Subfamily! Species Specimens hundreds of species awaiting description.! ! Aleocharinae! Many Many Two elements make the Green River Formation Omaliinae 2 3 fossils exciting: a) the limestone matrix is very fine ! and allowed for the preservation of minute details Oxytelinae! 6+ 27 of these fossils and b) the already described fossils support the hypothesis that the climate of Fig. 2 Staphylinites obsoletum Scudder! Fig. 3 Lathrobium abscessum Scudder! Fig. 4 Steninae! Paederinae! 8+ 94+ North America was much warmer 48 million years ago than it is today. The abovementioned fossils Pselaphinae! 2 2 point to a moist subtropical climate with average Staphylininae 4+ 10 annual temperatures ~4°C higher than today. -

Edible Insects and Other Invertebrates in Australia: Future Prospects
Alan Louey Yen Edible insects and other invertebrates in Australia: future prospects Alan Louey Yen1 At the time of European settlement, the relative importance of insects in the diets of Australian Aborigines varied across the continent, reflecting both the availability of edible insects and of other plants and animals as food. The hunter-gatherer lifestyle adopted by the Australian Aborigines, as well as their understanding of the dangers of overexploitation, meant that entomophagy was a sustainable source of food. Over the last 200 years, entomophagy among Australian Aborigines has decreased because of the increasing adoption of European diets, changed social structures and changes in demography. Entomophagy has not been readily adopted by non-indigenous Australians, although there is an increased interest because of tourism and the development of a boutique cuisine based on indigenous foods (bush tucker). Tourism has adopted the hunter-gatherer model of exploitation in a manner that is probably unsustainable and may result in long-term environmental damage. The need for large numbers of edible insects (not only for the restaurant trade but also as fish bait) has prompted feasibility studies on the commercialization of edible Australian insects. Emphasis has been on the four major groups of edible insects: witjuti grubs (larvae of the moth family Cossidae), bardi grubs (beetle larvae), Bogong moths and honey ants. Many of the edible moth and beetle larvae grow slowly and their larval stages last for two or more years. Attempts at commercialization have been hampered by taxonomic uncertainty of some of the species and the lack of information on their biologies. -

Zootaxa, Catalogue of Family-Group Names in Cerambycidae
Zootaxa 2321: 1–80 (2009) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2009 · Magnolia Press ISSN 1175-5334 (online edition) ZOOTAXA 2321 Catalogue of family-group names in Cerambycidae (Coleoptera) YVES BOUSQUET1, DANIEL J. HEFFERN2, PATRICE BOUCHARD1 & EUGENIO H. NEARNS3 1Agriculture and Agri-Food Canada, Central Experimental Farm, Ottawa, Ontario K1A 0C6. E-mail: [email protected]; [email protected] 2 10531 Goldfield Lane, Houston, TX 77064, USA. E-mail: [email protected] 3 Department of Biology, Museum of Southwestern Biology, University of New Mexico, Albuquerque, NM 87131-0001, USA. E-mail: [email protected] Corresponding author: [email protected] Magnolia Press Auckland, New Zealand Accepted by Q. Wang: 2 Dec. 2009; published: 22 Dec. 2009 Yves Bousquet, Daniel J. Heffern, Patrice Bouchard & Eugenio H. Nearns CATALOGUE OF FAMILY-GROUP NAMES IN CERAMBYCIDAE (COLEOPTERA) (Zootaxa 2321) 80 pp.; 30 cm. 22 Dec. 2009 ISBN 978-1-86977-449-3 (paperback) ISBN 978-1-86977-450-9 (Online edition) FIRST PUBLISHED IN 2009 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2009 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. -

A Contribution to the Knowledge of Tachyporiane Group of Rove Beetles
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Linzer biologische Beiträge Jahr/Year: 2011 Band/Volume: 0043_2 Autor(en)/Author(s): Samin Najmeh, Zhou Hong-Zhang, Sakenin Hamid, Imani Sohrab, Rastegar Jinoos Artikel/Article: A contribution to the knowledge of Tachyporiane group of rove beetles (Coleoptera: Staphylinoidea: Staphylinidae) from Iran 1579-1586 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Linzer biol. Beitr. 43/2 1579-1586 19.12.2011 A contribution to the knowledge of Tachyporiane group of rove beetles (Coleoptera: Staphylinoidea: Staphylinidae) from Iran N. SAMIN, H. ZHOU, H. SAKENIN, S. IMANI & J. RASTEGAR Abstract: One of the four staphylinid groups (Coleoptera: Staphylinidae), Tachyporiane group is studied in this paper. In a total of 24 species from 14 genera (including Phloeocharis MANNERHEIM, Bryophacis REITTER, Ischnosoma STEPHENS, Lordithon THOMSON, Mycetoporus MANNERHEIM, Sepedophilus GISTEL, Tachinus GRAVENHORST, Tachyporus GRAVENHORST, Aleochara GRAVENHORST, Atheta THOMSON, Myrmecopora SAULCY, Leptusa KRAATZ, Euryalea MULSANT & REY, Oxypoda MANNERHEIM) and 3 subfamilies (including, Phloeocharinae, Tachyporinae, Aleocharinae) are listed in this paper. Key words: Coleoptera, Staphylinidae, Tachyporiane Group, Fauna, Iran. Introduction Staphylinidae (Coleoptera) is one of the largest families of beetles, with over 46,000 species known worldwide (NEWTON et al. 2001). Rove beetles are known from every type of habitat that beetles occur in, and their diets include just about everything except the living tissues of higher plants. Most rove beetles are predators of insects and other kinds of invertebrates, living in forest leaf litter and similar kinds of decaying plant mat- ter. They are also commonly found under stones, and around freshwater margins. -

Coleoptera: Staphylinidae) Fauna of Turkey
_____________Mun. Ent. Zool. Vol. 4, No. 2, June 2009__________ 551 SOME ADDITIONAL NOTES ABOUT TACHYPORINAE (COLEOPTERA: STAPHYLINIDAE) FAUNA OF TURKEY Sinan Anlaş* and Armin Rose ** * Department of Biology, Faculty of Science, University of Ege, 35100 Bornova, İzmir / TURKEY. E-mail: [email protected] ** DZMB, German Centre for Marine Biodiversity Research, Senckenberg Research Institute, Südstrand 44, D-26382 Wilhelmshaven / GERMANY. E-mail: [email protected] [Anlaş, S. & Rose, A. 2009. Some additional notes about Tachyporinae (Coleoptera: Staphylinidae) fauna of Turkey. Munis Entomology & Zoology, 4 (2): 551-554] ABSTRACT: In this study, additional notes on 17 species of Tachyporinae new to certain Turkish provinces are given. Mycetoporus ignidorsum Eppelsheim, 1880 is the first record for the Turkish fauna. KEYWORDS: Staphylinidae, Tachyporinae, new record, fauna, Turkey. The staphylinid beetle subfamily Tachyporinae contains more than 1.500 known species out of 39 genera worldwide (Herman, 2001). According to Anlaş (2009), 80 species and subspecies of Tachyporinae have been reported for Turkey. However, many Turkish provinces are sparsely investigated regarding their Tachyporinae inventory. The aim of this study is to further enhance the knowledge on distibutions of Tachyporinae in Turkey. MATERIAL AND METHOD The present paper is based primarily on material collected during three field trips to Antalya carried out by Armin Rose in March 2000, 2001, and 2002, as well as recent collections by Sinan Anlaş and Ersen Aydın Yağmur. Classification and nomenclature of the Staphylinidae suggested by Herman (2001) and Löbl & Smetana (2004) has been followed in this study. The material referred to in this study is deposited in the following collections: LEMT …………………………….. -

Local and Landscape Effects on Carrion-Associated Rove Beetle (Coleoptera: Staphylinidae) Communities in German Forests
Supplementary Materials Local and Landscape Effects on Carrion-Associated Rove Beetle (Coleoptera: Staphylinidae) Communities in German Forests Sandra Weithmann1*, Jonas Kuppler1, Gregor Degasperi2, Sandra Steiger3, Manfred Ayasse1, Christian von Hoermann4 1 Institute of Evolutionary Ecology and Conservation Genomics, University of Ulm, 89069 Ulm, Germany; [email protected] (J.K.), [email protected] (M.A.) 2 Richard-Wagnerstraße 9, 6020 Innsbruck, Austria; [email protected] (G.D.) 3 Department of Evolutionary Animal Ecology, University of Bayreuth, 95447 Bayreuth, Germany; [email protected] (S.S.) 4 Department of Conservation and Research, Bavarian Forest National Park, 94481 Grafenau, Germany; [email protected] (C.v.H.) * Correspondence: [email protected] (S.W.) The supplementary material is structured in sections Materials and methods and Results. 1 Materials and methods Table S1: Environmental variables included in the analyses undertaken on the BExIS platform (Biodiversity Exploratories Information System, https://www.bexis.uni-jena.de). Variable Variable Categories or description of unit Data source information type variable region categorial Schwäbische Alb (ALB), Hainich- - ID: 20826, version: 1.14.10, Dün (HAI), Schorfheide-Chorin owner: Nieschulze, Schulze, (SCH) Fischer, Ayasse, Weisser, Ostrowski, König-Ries SMI index continuous silvicultural management intensity - ID: 17746, version: 1.2.2; (SMI) index (from 0 to 1) owner: Schall & Ammer 0 = undisturbed 1 = -

Newsletter #25.Pub
University of California, Riverside No. 26, Summer 2005 Friends of the Entomology Research Museum Newsletter Editor: Doug Yanega Proofing Editors: G. Ballmer, D. Hawks, R. Vetter FERM Officers FERM Annual Meeting! President : Alexis Park Vice-president: Ken Osborne Saturday, February 5th 2005 Treasurer: David Hawks Secretary: Marcella Waggoner 6:30 PM, UCR Entomology Building E-mails: [email protected], [email protected] [email protected], [email protected] Guest Speaker: Dr. Peter S. Cranston “Travels in Remote Gondwana: Midges in Deep Time” Editorial transition and apology No doubt it hasn’t escaped peoples’ attention that this news- letter is long overdue - that we had no Spring 2005 issue. Ea- It’s that time again! Our Annual gle-eyed regulars may also have noticed a slight shift in the th names at the top of the page, as well. Without going into grue- Meeting will be February 5 (Saturday) start- some details, our long-standing editor, Rick Vetter, has re- ing at 6:30 PM. Our speaker is Dr. Pete cently experienced a change in his position here at UCR, ne- Cranston, Professor of Entomology from the cessitating—at least temporarily—that he would be unable to University of California, Davis. Dr. Cran- work on the Newsletter. We were not really prepared for this ston teaches courses in systematic entomol- eventuality, and it’s taken some time to figure out what, ex- actly, our “Plan B” would be. So, for the foreseeable future, it ogy and biodiversity, and his research inter- looks like I, Doug Yanega, will have to take over the primary ests include the systematics, ecology, and Editorial duties, despite my glaring lack of familiarity with the biogeography of aquatic insects, particularly proper use of Microsoft Publisher. -

WORLD LIST of EDIBLE INSECTS 2015 (Yde Jongema) WAGENINGEN UNIVERSITY PAGE 1
WORLD LIST OF EDIBLE INSECTS 2015 (Yde Jongema) WAGENINGEN UNIVERSITY PAGE 1 Genus Species Family Order Common names Faunar Distribution & References Remarks life Epeira syn nigra Vinson Nephilidae Araneae Afregion Madagascar (Decary, 1937) Nephilia inaurata stages (Walck.) Nephila inaurata (Walckenaer) Nephilidae Araneae Afr Madagascar (Decary, 1937) Epeira nigra Vinson syn Nephila madagscariensis Vinson Nephilidae Araneae Afr Madagascar (Decary, 1937) Araneae gen. Araneae Afr South Africa Gambia (Bodenheimer 1951) Bostrichidae gen. Bostrichidae Col Afr Congo (DeFoliart 2002) larva Chrysobothris fatalis Harold Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) larva Lampetis wellmani (Kerremans) Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) syn Psiloptera larva wellmani Lampetis sp. Buprestidae Col jewel beetle Afr Togo (Tchibozo 2015) as Psiloptera in Tchibozo but this is Neotropical Psiloptera syn wellmani Kerremans Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) Psiloptera is larva Neotropicalsee Lampetis wellmani (Kerremans) Steraspis amplipennis (Fahr.) Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) larva Sternocera castanea (Olivier) Buprestidae Col jewel beetle Afr Benin (Riggi et al 2013) Burkina Faso (Tchinbozo 2015) Sternocera feldspathica White Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) adult Sternocera funebris Boheman syn Buprestidae Col jewel beetle Afr Zimbabwe (Chavanduka, 1976; Gelfand, 1971) see S. orissa adult Sternocera interrupta (Olivier) Buprestidae Col jewel beetle Afr Benin (Riggi et al 2013) Cameroun (Seignobos et al., 1996) Burkina Faso (Tchimbozo 2015) Sternocera orissa Buquet Buprestidae Col jewel beetle Afr Botswana (Nonaka, 1996), South Africa (Bodenheimer, 1951; syn S. funebris adult Quin, 1959), Zimbabwe (Chavanduka, 1976; Gelfand, 1971; Dube et al 2013) Scarites sp. Carabidae Col ground beetle Afr Angola (Bergier, 1941), Madagascar (Decary, 1937) larva Acanthophorus confinis Laporte de Cast. -

Titanus Fever
The business end of a 155mm male Titanus. Prionids in general are belligerent and care- less handling will result in a painful bite. The relatively short but very powerful mandibles of Titanus can cut pencils and biros in half and would be capable of inflicting serious damage to a human finger. Photograph by Simon Fearn. Titanus fever. An obsession with the world's largest beetle. By day, Simon Fearn is the Collection Officer - Natural Sciences for the Queen Victoria Museum and Art Gallery in Launceston. But in his spare time, he is compelled to indulge a consuming passion for anything vaguely - therapeutic for him, the amazing history of an extraordinary insect. efore my interest in reptiles came to the fore, I of my life, in 1974, that a chance encounter with an B was a kid obsessed with insects and spiders, old copy of The National Geographic Magazine in and I began an insect collection at a very early the school library completely blew my mind. age. I quickly developed a desire to understand the life cycles and ecology of the insects that I was Flipping through the May 1959 edition I came upon finding, but back in the early 1970s there was not a life-size depiction of a prionid longicorn beetle (on a great deal of information. At around this time the page 659), along with a huge grub which blanketed the page. The accompanying article was entitled published by the CSIRO and it became my bible. This allowed me to identify and classify many of it was my first introduction to the world's biggest the insects I was finding, but there were still insect - the aptly named Titanus giganteus. -

(Coleoptera: Staphylinidae) of South Carolina, Based on Published Records
The Coleopterists Bulletin, 71(3): 513–527. 2017. ACHECKLIST OF THE ROVE BEETLES (COLEOPTERA:STAPHYLINIDAE) OF SOUTH CAROLINA,BASED ON PUBLISHED RECORDS MICHAEL S. CATERINO AND MICHAEL L. FERRO Clemson University Arthropod Collection Department of Plant and Environmental Sciences 277 Poole Agricultural Center, Clemson University Clemson, SC 29634-0310, USA [email protected], [email protected] ABSTRACT A review of the literature revealed 17 subfamilies and 355 species of rove beetles (Staphylinidae) reported from South Carolina. Updated nomenclature and references are provided for all species. The goal of this list is to set a baseline for improvement of our knowledge of the state’s staphylinid fauna, as well as to goad ourselves and others into creating new, or updating existing, regional faunal lists of the world’s most speciose beetle family. Key Words: checklist, regional fauna, biodiversity, Nearctic DOI.org/10.1649/0010-065X-71.3.513 Staphylinidae, the rove beetles, are a megadiverse South Carolina is a rather small, yet diverse state, family of beetles containing more than 62,000 de- ranging from low-lying coastal habitats through a scribed species worldwide. The family is found in variety of mid-elevation communities to montane virtually all terrestrial habitats except in the extreme areas encompassing some of the diversity of higher polar regions. It is the most diverse family across all Appalachia. The easternmost portion of the state is animal groups. Within the Nearctic region (non- within the Atlantic Coastal Plain, a recently rec- tropical North America), about 4,500 species are ognized biodiversity hotspot (Noss 2016) that in- known (Newton et al. -

National Program 304 – Crop Protection and Quarantine
APPENDIX 1 National Program 304 – Crop Protection and Quarantine ACCOMPLISHMENT REPORT 2007 – 2012 Current Research Projects in National Program 304* SYSTEMATICS 1245-22000-262-00D SYSTEMATICS OF FLIES OF AGRICULTURAL AND ENVIRONMENTAL IMPORTANCE; Allen Norrbom (P), Sonja Jean Scheffer, and Norman E. Woodley; Beltsville, Maryland. 1245-22000-263-00D SYSTEMATICS OF BEETLES IMPORTANT TO AGRICULTURE, LANDSCAPE PLANTS, AND BIOLOGICAL CONTROL; Steven W. Lingafelter (P), Alexander Konstantinov, and Natalie Vandenberg; Washington, D.C. 1245-22000-264-00D SYSTEMATICS OF LEPIDOPTERA: INVASIVE SPECIES, PESTS, AND BIOLOGICAL CONTROL AGENTS; John W. Brown (P), Maria A. Solis, and Michael G. Pogue; Washington, D.C. 1245-22000-265-00D SYSTEMATICS OF PARASITIC AND HERBIVOROUS WASPS OF AGRICULTURAL IMPORTANCE; Robert R. Kula (P), Matthew Buffington, and Michael W. Gates; Washington, D.C. 1245-22000-266-00D MITE SYSTEMATICS AND ARTHROPOD DIAGNOSTICS WITH EMPHASIS ON INVASIVE SPECIES; Ronald Ochoa (P); Washington, D.C. 1245-22000-267-00D SYSTEMATICS OF HEMIPTERA AND RELATED GROUPS: PLANT PESTS, PREDATORS, AND DISEASE VECTORS; Thomas J. Henry (P), Stuart H. McKamey, and Gary L. Miller; Washington, D.C. INSECTS 0101-88888-040-00D OFFICE OF PEST MANAGEMENT; Sheryl Kunickis (P); Washington, D.C. 0212-22000-024-00D DISCOVERY, BIOLOGY AND ECOLOGY OF NATURAL ENEMIES OF INSECT PESTS OF CROP AND URBAN AND NATURAL ECOSYSTEMS; Livy H. Williams III (P) and Kim Hoelmer; Montpellier, France. * Because of the nature of their research, many NP 304 projects contribute to multiple Problem Statements, so for the sake of clarity they have been grouped by focus area. For the sake of consistency, projects are listed and organized in Appendix 1 and 2 according to the ARS project number used to track projects in the Agency’s internal database.