Panoramic Analysis on Ground Water and Landuse in Acharapakkam Mini Water Shed Using Arc Gis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Village Panchayats in Tamil Nadu District Code District Name
List of Village Panchayats in Tamil Nadu District Code District Name Block Code Block Name Village Code Village Panchayat Name 1 Kanchipuram 1 Kanchipuram 1 Angambakkam 2 Ariaperumbakkam 3 Arpakkam 4 Asoor 5 Avalur 6 Ayyengarkulam 7 Damal 8 Elayanarvelur 9 Kalakattoor 10 Kalur 11 Kambarajapuram 12 Karuppadithattadai 13 Kavanthandalam 14 Keelambi 15 Kilar 16 Keelkadirpur 17 Keelperamanallur 18 Kolivakkam 19 Konerikuppam 20 Kuram 21 Magaral 22 Melkadirpur 23 Melottivakkam 24 Musaravakkam 25 Muthavedu 26 Muttavakkam 27 Narapakkam 28 Nathapettai 29 Olakkolapattu 30 Orikkai 31 Perumbakkam 32 Punjarasanthangal 33 Putheri 34 Sirukaveripakkam 35 Sirunaiperugal 36 Thammanur 37 Thenambakkam 38 Thimmasamudram 39 Thilruparuthikundram 40 Thirupukuzhi List of Village Panchayats in Tamil Nadu District Code District Name Block Code Block Name Village Code Village Panchayat Name 41 Valathottam 42 Vippedu 43 Vishar 2 Walajabad 1 Agaram 2 Alapakkam 3 Ariyambakkam 4 Athivakkam 5 Attuputhur 6 Aymicheri 7 Ayyampettai 8 Devariyambakkam 9 Ekanampettai 10 Enadur 11 Govindavadi 12 Illuppapattu 13 Injambakkam 14 Kaliyanoor 15 Karai 16 Karur 17 Kattavakkam 18 Keelottivakkam 19 Kithiripettai 20 Kottavakkam 21 Kunnavakkam 22 Kuthirambakkam 23 Marutham 24 Muthyalpettai 25 Nathanallur 26 Nayakkenpettai 27 Nayakkenkuppam 28 Olaiyur 29 Paduneli 30 Palaiyaseevaram 31 Paranthur 32 Podavur 33 Poosivakkam 34 Pullalur 35 Puliyambakkam 36 Purisai List of Village Panchayats in Tamil Nadu District Code District Name Block Code Block Name Village Code Village Panchayat Name 37 -
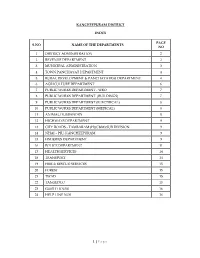
P Age KANCHEEPURAM DISTRICT INDEX S.NO NAME of THE
KANCHEEPURAM DISTRICT INDEX PAGE S.NO NAME OF THE DEPARTMENTS NO 1 DISTRICT ADMINISTRATION 2 2 REVENUE DEPARTMENT 2 3 MUNICIPAL ADMINISTRATION 3 4 TOWN PANCHAYAT DEPARTMENT 4 5 RURAL DEVELOPMENT & PANCHAYATRAJ DEPARTMENT 4 6 AGRICULTURE DEPARTMENT 6 7 PUBLIC WORKS DEPARTMENT - WRO 7 8 PUBLIC WORKS DEPARTMENT (BUILDINGS) 7 9 PUBLIC WORKS DEPARTMENT (ELECTRICAL) 8 10 PUBLIC WORKS DEPARTMENT (MEDICAL) 8 11 ANIMAL HUSBANDRY 8 12 HIGHWAYS DEPARTMENT 9 13 CITY ROADS - TAMBARAM (H)(C&M) SUB DIVISION 9 14 NHAI – PIU, KANCHEEPURAM 9 15 FISHERIES DEPARTMENT 9 16 POLICE DEPARTMENT 11 17 HEALTH SERVICES 14 18 TRANSPORT 14 19 FIRE & RESCUE SERVICES 15 20 FOREST 15 21 TWAD 15 22 TANGEDCO 15 23 GUEST HOUSE 16 24 HELP LINE NOS 16 1 | Page DISTRICT ADMINISTRATION Thiru.P.Ponniah, I.A.S., 044- 27237433 District Collector 044-27238477 9444134000 044-27238478 DRO (District Revenue Officer) 044-27237945 9445000903 044-27238995 Project Officer DRDA 044-27237153 7373704201 044-27238651 9443258833 044-27223353 Personal Assistant (General) 044-27237789 9445008138 044-27237909 Personal Assistant (Agriculture) 044-27237426 9444493040 Personal Assistant (Election) 044-27238445 9842503969 Personal Assistant (Accounts) 044-27237426 9600255568 7904127878 Special Deputy Collector (SSS) 044-27236623 9445461737 AC (Excise) 044-27237424 9942845207 DADWO (Adi Dravidar Welfare) 044-27236655 7338801259 DBCWO (Backward Class) 044-27236588 9443356133 DDAWO (Differently Abled) 044-27431853 9445497075 9445000168 District Supply Officer 044-27237424 9123555284 DSWO (Social Welfare) -

Kancheepuram Name Mobile Telephone Products Address Place Gnana Sekaran 9843769145 Sree Ramcides and Chemicals Jaya Prakash Agencies L
Kancheepuram Name Mobile Telephone Products Address Place Gnana Sekaran 9843769145 Sree Ramcides and chemicals Jaya prakash agencies L. Endathur Srinivasan 9865797553 Ramicides Sri Srinivasan Agro centre Acharapakkam G. Ramachandran 9597429111 Industrial India ltd Priya agro centre Acharapakkam C. Anandhan 9445139335 Ramcides Sri Amman Agro Acharapakkam D. Vasu 9443799672 Rallis INdia Saravana agri clinic Acharapakkam Anandasamy 8056624579 Tata Rallis Products Hi tech Agro Agencies Acharapakkam G. Ravi 9442409042 K.P.R. Fertilzers Sri Krishna Agro centre,Orathy Orathy K.Govindasamy and Chettiar and V.Selvaraj 9445124594 Anu products Orathy Sons G. Saraswathy 9442537854 Anu products Sri Rama Agro centre Orathy N. Thiagarajan 9445782365 Anu products Balaji Agencies Elapakkam S. Maharani 9445760997 Rallis INdia Bharani Agencies Elapakkam Ganapathy 9751087089 Makhetesim Agan Pvt ltd Sri Ganapathy Agro centre Elapakkam Jayalakshmi 9444511171 Ramcides Jayalakshmi Agro centre Madhur Melnatham Jayaraman 9443045447 Rallis India Ltd Gokul Agro centre Ramapuram Sri Krishna Agro centre, A.Gopal 9444692488 Rallis India Ramapuram Ramapuram ADA/AEC 9894215521 4427523411 All products AEC, Acharapakkam Acharapakkam ADA/AEC 9894215521 4427523411 All products Orathy Orathy ADA/AEC 9894215521 4427523411 All products L. Endathur L. Endathur D. Elumalai 9789372907 Ramcides Sri Kanna Agro centre Kadamalaiputhur V.S.Ganesan 9791313217 Sree Ramcides and chemicals V.S.GAnesh Agencies Kadamalaiputhur S. Ravikumar 9445630562 Anu products USHA agencies Ramapuram -

Branch Libraries List
ADDRESS OF BRANCH LIBRARIES 1 District Central Library, 16 Branch Library, 307, Anna Salai, 2D, Nadu Street, Chengalpet – 603 002. Achirupakkam – 603 301. 2 Branch Library, 17 Branch Library, 78, Station Road, Main Road, Kattangolathur – 603 203. Thozhupedu – 603 310. 3 Branch Library, 18 Branch Library, Gandhi Street, Main Road, Guduvancheri – 603 202. Orathy – 603 307. 4 Branch Library, 19 Branch Library, 2/45, B. Santhaimedu, Ladakaranai, Endathur, Singaperrumal Koil – 603 204. Uthiramerur – 603 406. 5 Branch Library, 20 Branch Library, 129, Thiruvalluvar Salai, Bajanai Koil Street, Maraimalai Nagar – 603 209. Elapakkam – 603 201. 6 Branch Library, 21 Branch Library, 5, West Mada Street, 5/55, Salt Road, Thiruporur – 603 110. Cheyyur – 603 202. 7 Branch Library, 22 Branch Library, 34, Mamallapuram Salai, Angalamman Koil Street, Thirukazhukundram – 603 109. Kuvathur – 603 305. 8 Branch Library, 23 Branch Library, 203, Kulakarai Street, 2, East Coast Road, Sembakkam – 603 108. Kadapakkam – 603 304. 9 Branch Library, 24 Branch Library, 105, W2, Brahmanar Street, 9, Chakkaram Kodhandarama P.V. Kalathur – 603 405. Iyengar Street, Uthiramerur – 603 406. 10 Branch Library, 25 Branch Library, East Raja Street, Hospital Road, Mamallapuram – 603 104. Kaliyampoondi – 603 403. 11 Branch Library, 26 Branch Library, Nesco Joint, 1/172, Road Street, Kalpakkam – 603 102. Manampathi – 603 403. 12 Branch Library, 27 Branch Library, 70, Car Street, Main Road, Madhuranthagam – 603 306. Perunagar – 603 404. 13 Branch Library, 28 Branch Library, 3, Othavadai Street, Perumal Koil Street, Karunguzhi – 603 303. Salavakkam – 603 107. 14 Branch Library, 29 Branch Library, Railway Station Road, 138, Pillaiyar Koil Street, Padalam – 603 308. -

Kancheepuram District Map N
KANCHEEPURAM DISTRICT MAP N W E TIRUVALLUR DISTRICT S THIRUVALLUR KONDAVAKKAM CHENNAI AYYAPANTHANGAL CHENNAI KANDAMANGALAM CHENNAI THELLIARAGARAM MEVALURCUPPAM SRINIVASAPURAM DISTRICT MANGADU T.P KOLATHUVANJERI NANDAMBAKKKAM SENGADU CHENNAI MANNUR KODAMANIVAKKAM BIT-3 CHINNA- MOWLIVAKKAM MUGALIVAKKAM PANICHERI PARANIPUTHUR SIVABURAM MALAYAMBAKKAM MADANANDAPURAM NANDAMBAKKAM VALARPURAM PERIA- BIT-1 CHENNAI ORAGADAMAHADEVINANGALAM PANICHER GERUGAMBAKKAM CHEMBARAMBAKKAM TANK SIKKARAYAPURAM KOVUR NANDAMBAKKAM T.P ALANDUR MANAPAKKAM KAPPANKOTTUR THANDALAM KOLACHERY COWL BAZAAR KUNNATHUR bit2 MOONRAM- ST.THOMAS MOUNT PICHIVAKKAM THANDALAM THIRUNAGESWARAM KATTALAI TALUK NANDAMBAKKAM CHENNAI NEMEMLI THANDALAM UDUPPAIR BIT-2 KANDIVAKKAM ELIMIANKDTTUR R.F SIRUKALATHUR KAVANUR RENDANKATTALAI VENKATEPURAM IRUNGATTUKOTTAI THARAPPAKKAM COWL BAZAAR PALAVANTANGAL KOTTUR MANANJERI (PART) THOLASAPURAM THARVUR POLICHALUR MEENAMBAKKAM KILOY T.P AYAKOLATHUR ALANDUR KUNNATHUR T.P MUNICIPALITY PURISAI EDAYARPAKKAM KATTRAMBAKKAM PALLAVARAM GUNAGARABAKKAM ETTIKUTHIMEDU ANAKAPUTHUR CONTONMENT CHENNAI NANDAMBAKKAM KUNRATHUR T.P PENNALUR ULLAGARAM T.M SIRUKILOY THIRUSOLAM KANAGAMBAKKAM THANDALAM AKKAMAPURAM MAHADEVIMANGALAM PUDUPPAIR KOTTIVAKKAM PADICHERY THIRUMUDIVAKKAM PAMMAL T.P KANNANTHANGAL POONTHANDALAM MOOVARASAMPATTU PERUNGUDI T.P PULLALORE PALLAMBAKKAM SRIPERUMBUDUR T.P AMARAMBEDU R.F PALLAVARAM PALVAKKAM ARAKONAM VALATHUR EKANAPURAM MADIPAKKAM (PART) PALANTHANDALAM MALLUR VELLORE SINGLEPADI MADURAMANGALAM VADAMANGALAM VENGADU NALLUR PADUNALLI PONDAVAKKAM -

Chengalpattu District
DISTRICT DISASTER MANAGEMENT PLAN 2020 CHENGALPATTU DISTRICT District Disaster Management Authority Chengalpattu District, Tamil Nadu DISTRICT DISASTER MANAGEMENT PLAN 2020 DISTRICT DISASTER MANAGEMENT AUTHORITY CHENGALPATTU DISTRICT TAMIL NADU PREFACE Endowed with all the graces of nature’s beauty and abundance, the newly created district of Chengalpattu is a vibrant administrative entity on the North eastern part of the state of Tamil Nadu. In spite of the district’s top-notch status in terms of high educational, human development index and humungous industrial productivity, given its geography, climate and certain other socio-political attributes, the district administration and its people have to co-exist with the probabilities of hazards like floods, cyclone, Tsunami, drought, heat wave, lightning and chemical, biological, radiological and nuclear emergencies. The Disastrous events in the recent past like the Tsunami of 2004, the catastrophic floods of year 2015, the cyclone of year 2016 and most recently the COVID-19 pandemic, will serve as a testament to the district’s vulnerability to such hazards. How the society responds to such vagaries of nature decides the magnitude and intensity of the destruction that may entail hazardous events. It is against this back drop, the roll of the District Disaster Management Authority can be ideally understood. The change in perspective from a relief- based approach to a more holistic disaster management approach has already begun to gain currency among the policy makers due to its substantial success in efficient handling of recent disasters across the globe. The need of the hour, therefore, is a comprehensive disaster management plan which is participative and people-friendly with the component of inter- departmental co-ordination at its crux. -

Analysis of Road Traffic Accidents on NH45, Kanchipuram District (Tamil Nadu, India)
4th IRTAD CONFERENCE 16-17 September, 2009, Seoul, Korea Analysis of Road Traffic Accidents on NH45, Kanchipuram District (Tamil Nadu, India) Ravishankar Rajaraman INDIA, JP Research, [email protected] ABSTRACT With the support and cooperation of the Kanchipuram district police and Tamil Nadu police, researchers conducted detailed investigations of accidents occurring on the National Highway 45 over a 60 km stretch. The primary objective was to collect and analyze India-based traffic crash data to begin to create a sound basis for decision making for improving safety on India’s roadways. A secondary objective was to establish a standardized methodology for collecting and analyzing crash data, specific to Indian roads. For the 45 day study period, an accident intimation network was established between researchers and all police stations/highway patrols in the study area. On occurrence of an accident, police called a 24-hour contact number and researchers responded to the scene. On site, researchers used standardized reporting forms, methodologies, and equipment to perform accident scene examinations, accident vehicle examination, and AIS injury coding. The collected accident data was categorized first by single- or multiple-vehicle crash and next by accident type based on the first accident event. The data was then analyzed to identify accident (crash type, location time), vehicle (vehicle type, pre-crash condition), occupant (restraint use, gender, age) and other contributing factors, and environmental factors associated with injury severity. Findings show that front-to-rear collisions, mainly involving heavy trucks and buses, caused due to slowing down, stopping, breaking down or overtaking account for 59% of the accidents. -

Kanchipuram District
DISTRICT SURVEY REPORT FOR MINOR MINERALS KANCHEEPURAM DISTRICT Prepared as per the Ministry of Environment, Forest and Climate Change MoEF & CC Gazette Notification S.O.3611 (E) Dated 25.07.2018. MAY 2019 DISTRICT SURVEY REPORT KANCHEEPURAM DISTRICT Chapter Content Page No. 1 Introduction 1 2 Overview of Mining Activity in the District 4 3 General Profile of The District 5 4 Geology and Mineral wealth of the district 10 5 Drainage of Irrigation Pattern 24 6 Land utilisation pattern in the district 25 7 Surface Water And Ground Water Scenario of The 28 District 8 Rainfall Of The District And Climatic Condition 31 9 Details Of The Mining Quarry leases In The 33 Kancheepuram District 10 Details of Royalty or Revenue Received in Last 35 Three Years 11 Details of Production of Minor Mineral in Last Three 38 Years 12 Mineral Map of The District 38 13 List of Letter of Intent (LOI) Holders in The District 39 Along With it’s Validity 14 Total Mineal Reserve Available in The District 39 15 Quality / Grade of Mineral available in the District 39 16 Use of Mineral 39 17 Demand and Supply of the Mineral in the last three 40 years 18 Mining Leases marked on the map the district 40 i 19 Details of the area of where there is a cluster of 41 mining leases viz number of mining leases, location (latitude and longitude) 20 Details of Eco-Sensitive Area 41 21 Impact on the Environment (Air, Water, Soil, Flora 42 & Fauna, land use, agriculture, forest etc.) due to mining activity: 22 Remedial Measures to mitigate the impact of 43 mining on the Environment 23 Reclamation of Mined out area (best practice 43 already implemented in the district, requirement as per rules and regulation, proposed reclamation plan) 24 Risk Assessment & Disaster Management Plan: 44 25 Details of the Occupational Health issues in the 48 District. -
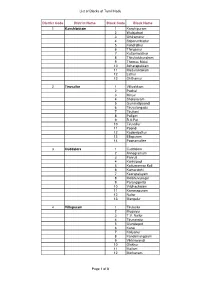
List of Blocks of Tamil Nadu District Code District Name Block Code
List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 1 Kanchipuram 1 Kanchipuram 2 Walajabad 3 Uthiramerur 4 Sriperumbudur 5 Kundrathur 6 Thiruporur 7 Kattankolathur 8 Thirukalukundram 9 Thomas Malai 10 Acharapakkam 11 Madurantakam 12 Lathur 13 Chithamur 2 Tiruvallur 1 Villivakkam 2 Puzhal 3 Minjur 4 Sholavaram 5 Gummidipoondi 6 Tiruvalangadu 7 Tiruttani 8 Pallipet 9 R.K.Pet 10 Tiruvallur 11 Poondi 12 Kadambathur 13 Ellapuram 14 Poonamallee 3 Cuddalore 1 Cuddalore 2 Annagramam 3 Panruti 4 Kurinjipadi 5 Kattumannar Koil 6 Kumaratchi 7 Keerapalayam 8 Melbhuvanagiri 9 Parangipettai 10 Vridhachalam 11 Kammapuram 12 Nallur 13 Mangalur 4 Villupuram 1 Tirukoilur 2 Mugaiyur 3 T.V. Nallur 4 Tirunavalur 5 Ulundurpet 6 Kanai 7 Koliyanur 8 Kandamangalam 9 Vikkiravandi 10 Olakkur 11 Mailam 12 Merkanam Page 1 of 8 List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 13 Vanur 14 Gingee 15 Vallam 16 Melmalayanur 17 Kallakurichi 18 Chinnasalem 19 Rishivandiyam 20 Sankarapuram 21 Thiyagadurgam 22 Kalrayan Hills 5 Vellore 1 Vellore 2 Kaniyambadi 3 Anaicut 4 Madhanur 5 Katpadi 6 K.V. Kuppam 7 Gudiyatham 8 Pernambet 9 Walajah 10 Sholinghur 11 Arakonam 12 Nemili 13 Kaveripakkam 14 Arcot 15 Thimiri 16 Thirupathur 17 Jolarpet 18 Kandhili 19 Natrampalli 20 Alangayam 6 Tiruvannamalai 1 Tiruvannamalai 2 Kilpennathur 3 Thurinjapuram 4 Polur 5 Kalasapakkam 6 Chetpet 7 Chengam 8 Pudupalayam 9 Thandrampet 10 Jawadumalai 11 Cheyyar 12 Anakkavoor 13 Vembakkam 14 Vandavasi 15 Thellar 16 Peranamallur 17 Arni 18 West Arni 7 Salem 1 Salem 2 Veerapandy 3 Panamarathupatti 4 Ayothiyapattinam Page 2 of 8 List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 5 Valapady 6 Yercaud 7 P.N.Palayam 8 Attur 9 Gangavalli 10 Thalaivasal 11 Kolathur 12 Nangavalli 13 Mecheri 14 Omalur 15 Tharamangalam 16 Kadayampatti 17 Sankari 18 Idappady 19 Konganapuram 20 Mac. -

List of Town Panchayats Name in Tamil Nadu Page 1 District Code
List of Town Panchayats Name in Tamil Nadu Sl. No. District Code District Name Town Panchayat Name 1 1 KANCHEEPURAM ACHARAPAKKAM 2 1 KANCHEEPURAM CHITLAPAKKAM 3 1 KANCHEEPURAM EDAKALINADU 4 1 KANCHEEPURAM KARUNGUZHI 5 1 KANCHEEPURAM KUNDRATHUR 6 1 KANCHEEPURAM MADAMBAKKAM 7 1 KANCHEEPURAM MAMALLAPURAM 8 1 KANCHEEPURAM MANGADU 9 1 KANCHEEPURAM MEENAMBAKKAM 10 1 KANCHEEPURAM NANDAMBAKKAM 11 1 KANCHEEPURAM NANDIVARAM - GUDUVANCHERI 12 1 KANCHEEPURAM PALLIKARANAI 13 1 KANCHEEPURAM PEERKANKARANAI 14 1 KANCHEEPURAM PERUNGALATHUR 15 1 KANCHEEPURAM PERUNGUDI 16 1 KANCHEEPURAM SEMBAKKAM 17 1 KANCHEEPURAM SEVILIMEDU 18 1 KANCHEEPURAM SHOLINGANALLUR 19 1 KANCHEEPURAM SRIPERUMBUDUR 20 1 KANCHEEPURAM THIRUNEERMALAI 21 1 KANCHEEPURAM THIRUPORUR 22 1 KANCHEEPURAM TIRUKALUKUNDRAM 23 1 KANCHEEPURAM UTHIRAMERUR 24 1 KANCHEEPURAM WALAJABAD 25 2 TIRUVALLUR ARANI 26 2 TIRUVALLUR CHINNASEKKADU 27 2 TIRUVALLUR GUMMIDIPOONDI 28 2 TIRUVALLUR MINJUR 29 2 TIRUVALLUR NARAVARIKUPPAM 30 2 TIRUVALLUR PALLIPATTU 31 2 TIRUVALLUR PONNERI 32 2 TIRUVALLUR PORUR 33 2 TIRUVALLUR POTHATTURPETTAI 34 2 TIRUVALLUR PUZHAL 35 2 TIRUVALLUR THIRUMAZHISAI 36 2 TIRUVALLUR THIRUNINDRAVUR 37 2 TIRUVALLUR UTHUKKOTTAI Page 1 List of Town Panchayats Name in Tamil Nadu Sl. No. District Code District Name Town Panchayat Name 38 3 CUDDALORE ANNAMALAI NAGAR 39 3 CUDDALORE BHUVANAGIRI 40 3 CUDDALORE GANGAIKONDAN 41 3 CUDDALORE KATTUMANNARKOIL 42 3 CUDDALORE KILLAI 43 3 CUDDALORE KURINJIPADI 44 3 CUDDALORE LALPET 45 3 CUDDALORE MANGALAMPET 46 3 CUDDALORE MELPATTAMPAKKAM 47 3 CUDDALORE PARANGIPETTAI -
CIN/BCIN Company/Bank Name
Note: This sheet is applicable for uploading the particulars related to the unclaimed and unpaid amount pending with company. Make sure that the details are in accordance with the information already provided in e-form IEPF-2 CIN/BCIN L24110MH1947PLC005719 Prefill Company/Bank Name PIRAMAL ENTERPRISES LIMITED Date Of AGM(DD-MON-YYYY) 30-Jul-2018 Sum of unpaid and unclaimed dividend 12034164.00 Sum of interest on matured debentures 0.00 Sum of matured deposit 0.00 Sum of interest on matured deposit 0.00 Sum of matured debentures 0.00 Sum of interest on application money due for refund 0.00 Sum of application money due for refund 0.00 Redemption amount of preference shares 0.00 Sales proceed for fractional shares 0.00 Validate Clear Proposed Date of Investor First Investor Middle Investor Last Father/Husband Father/Husband Father/Husband Last DP Id-Client Id- Amount Address Country State District Pin Code Folio Number Investment Type transfer to IEPF Name Name Name First Name Middle Name Name Account Number transferred (DD-MON-YYYY) 39A SECOND STREET SMS LAYOUT PIRA000000000BS00 Amount for unclaimed and A ALAGIRI SWAMY NA ONDIPUTHUR COIMBATORE INDIA Tamil Nadu 641016 076 unpaid dividend 300.00 09-Sep-2018 H NO 6-3-598/51/12/B IST FLR ANAND NAGAR COLONY PIRA000000000AS01 Amount for unclaimed and A AMARENDRA NA HYDERABAD INDIA Andhra Pradesh 500004 467 unpaid dividend 300.00 09-Sep-2018 7-1-28/1/A/12 PARK AVENUE PIRA000000000AS01 Amount for unclaimed and A ANJI REDDY NA AMEERPET HYDERABAD INDIA Andhra Pradesh 500016 053 unpaid dividend 1200.00 09-Sep-2018 4/104, BOMMAIKUTTAI MEDU IN300159-10771263- Amount for unclaimed and A ARIVUCHELVAN NA SELLAPPAMPATTY P. -

I Mord Share State Share Total Cost 1 Kanchipuram Acharapakkam
TAMIL NADU STATE PMGSY II (2017-18)- ROAD WORKS ANNEXURE - I Construction Cost Maintenance Sl. Length District Block Road Name ( Rs.in lakh) Cost No (in Km.) MoRD Share State Share Total Cost ( Rs.in lakh) 1 Kanchipuram Acharapakkam MRL01-Karikylie to vellaputhur Road 4.000 52.79 49.50 102.29 14.40 MRL09-kiazhanivakkam to Muniyanthangal 2 Kanchipuram Acharapakkam 4.200 88.09 71.28 159.37 15.12 Road 3 Kanchipuram Acharapakkam MRL06-L.Endathur to Vaipanai Road 3.550 53.98 47.42 101.40 12.78 4 Kanchipuram Acharapakkam MRL10-MTD Road to Muniyanthangal Road 4.400 48.20 41.95 90.15 15.84 MRL18-OA Road to Vepangaranai 5 Kanchipuram Acharapakkam 3.300 45.65 39.18 84.83 11.88 Vinnampoondi Road 6 Kanchipuram Acharapakkam MRL22-Anaikunnam to Ezhupakkam road 2.550 25.75 24.14 49.89 9.18 7 Kanchipuram Chithamur MRL15-A.K punjai To Kayapakkam 3.450 106.02 78.30 184.32 12.42 MRL16-APK Road To CP road (via) 8 Kanchipuram Chithamur 7.100 184.61 138.61 323.22 25.56 Ammanampakkam MRL08-SAF Road To Puthupettai 9 Kanchipuram Chithamur 2.990 89.84 67.52 157.36 10.76 (via)Vellakondagaram TAMIL NADU STATE PMGSY II (2017-18)- ROAD WORKS ANNEXURE - I Construction Cost Maintenance Sl. Length District Block Road Name ( Rs.in lakh) Cost No (in Km.) MoRD Share State Share Total Cost ( Rs.in lakh) 10 Kanchipuram Chithamur T11-CP ROAD TO Thiruvalarcheri 3.450 127.21 92.95 220.16 12.42 MRL24-Musaravakkam Colony Kilar colony 11 Kanchipuram Kancheepuram 3.140 58.04 47.31 105.35 11.30 Road 12 Kanchipuram Kancheepuram MRL09-Kilperamanallur to Vedal Road 2.335 40.35 31.37