Sexually Antagonistic Coevolution in Insects Is Associated with Only Limited Morphological Diversity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Working List of Prairie Restricted (Specialist) Insects in Wisconsin (11/26/2015)
Working List of Prairie Restricted (Specialist) Insects in Wisconsin (11/26/2015) By Richard Henderson Research Ecologist, WI DNR Bureau of Science Services Summary This is a preliminary list of insects that are either well known, or likely, to be closely associated with Wisconsin’s original native prairie. These species are mostly dependent upon remnants of original prairie, or plantings/restorations of prairie where their hosts have been re-established (see discussion below), and thus are rarely found outside of these settings. The list also includes some species tied to native ecosystems that grade into prairie, such as savannas, sand barrens, fens, sedge meadow, and shallow marsh. The list is annotated with known host(s) of each insect, and the likelihood of its presence in the state (see key at end of list for specifics). This working list is a byproduct of a prairie invertebrate study I coordinated from1995-2005 that covered 6 Midwestern states and included 14 cooperators. The project surveyed insects on prairie remnants and investigated the effects of fire on those insects. It was funded in part by a series of grants from the US Fish and Wildlife Service. So far, the list has 475 species. However, this is a partial list at best, representing approximately only ¼ of the prairie-specialist insects likely present in the region (see discussion below). Significant input to this list is needed, as there are major taxa groups missing or greatly under represented. Such absence is not necessarily due to few or no prairie-specialists in those groups, but due more to lack of knowledge about life histories (at least published knowledge), unsettled taxonomy, and lack of taxonomic specialists currently working in those groups. -

Antagonistic Coevolution Between the Sexes in a Group of Insects
letters to nature Acknowledgements a central process of evolution, with the potential to shape various We thank the authorities in Madagascar and the Seychelles for permission to conduct interactions between the sexes2,5,6 and their gametes7±8, as well as ®eldwork. We thank J. Brown, T. Peterson, R. Prum, O. Rieppel, L. Trueb and E. Wiley for diversi®cation9, speciation and extinction rates10±12. At the core of comments. C.J.R. and R.A.N. were supported by the National Geographic Society and the this coevolutionary interaction is an arms race between the sexes National Science Foundation, Washington. that can include periods of both escalation and de-escalation of arms13±15. Competing interests statement Theory suggests, however, that the outcome of antagonistic The authors declare that they have no competing ®nancial interests. male±female interactions should remain relatively unchanged Correspondence and requests for materials should be addressed to C.J.R. during an arms race because the build-up of arms in one sex may (e-mail: [email protected]). Sequences are deposited of GenBank under accession numbers be balanced by a build-up in the other (Fig. 1a, 1±2). The AF443224±AF443275. consequences of such arms races on sexual interactions may thus be undetectable, which makes sexually antagonistic coevolution inherently dif®cult to show1±4. Perhaps for this reason, we have no direct empirical evidence for a primary role of arms races in the ................................................................. evolution of sexual interactions in natural systems. Despite an expectation of some evolutionary balance in the level Antagonistic coevolution between of arms between the sexes, one sex may at least temporarily evolve a greater quantity of arms relative to the other (refs 13±15; and Fig. -

A Check List of Gerromorpha (Hemiptera) from India
Rec. zool. Surv. India: 100 (Part 1-2) : 55-97, 2002 A CHECK LIST OF GERROMORPHA (HEMIPTERA) FROM INDIA G. THIRUMALAI Zoological Survey of India, Southern Regional Station, Chennai 600 028. INTRODUCflON The Infraorder Gerromorpha comprises of semi-aquatic bugs characterised by long conspicuous antennae, longer than head and inserted in front of eyes. They are distributed in all kinds of climatic zones, except the coldest and driest parts. This infraorder contains 8 families namely, Gerridae, Veliidae, Hydrometridae, Mesoveliidae, Hebridae, Macroveliidae, Paraphrynoveliidae and Hennatobatidae (Andersen, 1982a). Knowledge of Indian semi-aquatic Hemiptera is limited to the taxonomic preliminaries, recording species from different parts of the country. (Distant, 1903a; 1910 a&b; Annandale, 1919; Bergroth, 1915a; Paiva, 1919a & b; Dover, 1928; Hafiz & Mathai, 1938; Hafiz & Riberio, 1939; Hafiz & Pradhan, 1947; Pradhan, 1950a, b& 1975; Gupta, 1981; Selvanayagam, 1981; Roy et al. 1988; Ghosh et al. 1989; Polhenlus & Starmuhlner, 1990; Bal & Basu, 1994 & 1997; Chen & Zettel, 1999). The revisionary work of Andersen (1975, 1980, 1990 & 1993); Den Boer (1969); Hungerford & Matsuda (1958a & b, 1960, 1962b & 1965); Herring (1961); Polhemus & Andersen (1984); Andersen & Foster (1992); Andersen & Chen (1993); Chen & Nieser (1993a & b); Polhemus & Karunaratne (1993); Polhemus (1994); Polhemus & Polhemus (1994 & 1995a) on a few genera of Gerridae; Lundblad (1936) on the genera Rhagovelia Mayr and Tetraripis Lundblad; Andersen (1981 b, 1983 & 1989); Polhemus -

Fitting Together: Copulatory Linking in Some Neotropical Chrysomeloidea
Fitting together: copulatory linking in some Neotropical Chrysomeloidea R. Wills Flowers1 & William G. Eberhard2 1 Center for Biological Control, Florida A&M University, Tallahassee, FL 32307 USA; [email protected] 2 Escuela de Biología, Universidad de Costa Rica, 2060 San José, Costa Rica; [email protected] Received 21-VII-2005. Corrected 12-VIII-2005. Accepted 29-III-2005. Abstract: Copulatory linking of male and female genitalic structures in 11 Neotropical species of Chrysomelidae and one species of Megalopodidae was studied by freezing and then dissecting pairs of beetles in copula. In Megalopus armatus (Megalopodidae) the male has a long endophallus with complex membranous protuberances and a terminal flagellum that probably reaches the spermatheca. In the subfamily Eumolpinae the females have telescoping ovipositors through which the male endophalli pass, reaching to or near the mouth of the spermathe- cal duct. A long thin flagellum is probably inserted into the spermathecal duct. The male endophalli are braced inside the female using various structures, including two pairs of lateral appendages and apical appendages (both lateral pairs sclerotized in Colaspis sanjoseana and only the basal pair in Brachypnoea irazuensis), a pair of membranous swellings (in Metaxyonycha amasia), and apical microspicules on the endophallus (in Xanthonia). In the subfamily Galerucinae, males of Metrioidea and Diabrotica (tribe Galerucini) have relatively short endophalli ornamented with sclerotized hooks, spines and needles. In Metrioidea elongata the long needle-like endophallic spines of the male were erected inside the female and penetrated the wall of her bursa. In the tribe Alticini, the male endophallus is very short and does not enter the female in two species, Alagoasa gemmata and Walterianella sp. -

Sexually Antagonistic Coevolution in a Mating System
Sexually Antagonistic Coevolution in a Mating System: Combining Experimental and Comparative Approaches to Address Evolutionary Processes Author(s): Locke Rowe and Göran Arnqvist Reviewed work(s): Source: Evolution, Vol. 56, No. 4 (Apr., 2002), pp. 754-767 Published by: Society for the Study of Evolution Stable URL: http://www.jstor.org/stable/3061658 . Accessed: 01/10/2012 15:38 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Society for the Study of Evolution is collaborating with JSTOR to digitize, preserve and extend access to Evolution. http://www.jstor.org Evoluition, 56(4), 2002, pp. 754-767 SEXUALLY ANTAGONISTIC COEVOLUTION IN A MATING SYSTEM: COMBINING EXPERIMENTAL AND COMPARATIVE APPROACHES TO ADDRESS EVOLUTIONARY PROCESSES LOCKE ROWE"223 AND GORAN ARNQVIST4 'Department of Zoology, University of Toronto, Toronto, Ontario M5S 3G5, Canada 2Centre for Biodiversity and Conservation Biology, Royal Ontario Museum, Toronto, Ontario M5S 2C6 Canada 3E-mail: [email protected] 4Department of Animal Ecology, Evolutionary Biology Centre, University of Uppsala, Norbyvagen 18d, SE-752 36 Uppsala, Sweden Abstract.-We combined experimental and comparative techniques to study the evolution of mating behaviors within in a clade of 15 water striders (Gerris spp.). -

Coleoptera, Chrysomelidae, Galerucinae)
A peer-reviewed open-access journal ZooKeys 720:Traumatic 77–89 (2017) mating by hand saw-like spines on the internal sac in Pyrrhalta maculicollis 77 doi: 10.3897/zookeys.720.13015 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Traumatic mating by hand saw-like spines on the internal sac in Pyrrhalta maculicollis (Coleoptera, Chrysomelidae, Galerucinae) Yoko Matsumura1, Haruki Suenaga2, Yoshitaka Kamimura3, Stanislav N. Gorb1 1 Department of Functional Morphology and Biomechanics, Zoological Institute, Kiel University, Am Botani- schen Garten 1-9, D-24118 Kiel, Germany 2 Sunshine A205, Nishiachi-chô 833-8, Kurashiki-shi, Okayama Pref., 710-0807, Japan 3 Department of Biology, Keio University, 4-1-1 Hiyoshi, Yokohama 223-8521, Japan Corresponding author: Yoko Matsumura ([email protected]) Academic editor: Michael Schmitt | Received 1 April 2017 | Accepted 13 June 2017 | Published 11 December 2017 http://zoobank.org/BCF55DA6-95FB-4EC0-B392-D2C4B99E2C31 Citation: Matsumura Y, Suenaga H, Kamimura Y, Gorb SN (2017) Traumatic mating by hand saw-like spines on the internal sac in Pyrrhalta maculicollis (Coleoptera, Chrysomelidae, Galerucinae). In: Chaboo CS, Schmitt M (Eds) Research on Chrysomelidae 7. ZooKeys 720: 77–89. https://doi.org/10.3897/zookeys.720.13015 Abstract Morphology of the aedeagus and vagina of Pyrrhalta maculicollis and its closely related species were inves- tigated. The internal sac of P. maculicollis bears hand saw-like spines, which are arranged in a row. Healing wounds were found on the vagina of this species, whose females were collected in the field during a repro- ductive season. However, the number of the wounds is low in comparison to the number of the spines. -

Orthoptera Recording Scheme for Britain and Ireland
ORTHOPTERA RECORDING SCHEME FOR BRITAIN AND IRELAND Newsletter 25 - February 1999 Editor: John Widgery 2I FieldYiew Road Potters Bar Herts EN6 2NA Tel: 01707 642708 INTRODUCTION It seems incredible that another year has passed since the last newsletter (NL24). This current newsletter is inænded to update all readers of the most significant developments since then. Of course, those of you who take British Wildlife magazine may already be awarg tlrough my 'rWildlife Notes', of some of the information contained herein. The success ofthe scheme relies upon your endeavours and, once again, I am indebted to the many of you who have submitted records and also to Paul Pearce-Kelly, Rachel Jones and Bryan Pinchen for their contributions on rare species. SUMMARY OF HIGHLIGHTS In comparison with recent years, the summer of 1998 was disappointing, although parts of southern England did have some reÍlsonably warm and dry weather during August and early September which is probably the most important period for the breeding success of many species. It was, perhaps, not surprising that there were fewer records submitted during 1998 as compared with the previous yàr but, even so, there were still several thousand which involved a total of 349 new l0hn squares (including 68 post-1970 refinds). Of these, 195 (including 23 post-1970s) were for 1998, including first ever records for Roesel's Bush Cricket, Metrioptera roeselii, in the Channel Islands, Long-winged Conehead, Conocephalus discolor, in Cambridgeshire and Lesnets Earwig, Forfcula lesnei,in Worcestershire and also a national first for this latter species in lreland. Additionally, we had the most northerly yet records for Lesser Marsh Grasshopper, Chorthippus albomarginqtus. -

Systematics, Historical Biogeography and Ecological Phylogenetics in A
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Damgaard Jakob Artikel/Article: Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders 813-822 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders1 J. DAMGAARD Abstract: I hereby review the current knowledge about systematics, historical biogeography and ecolo- gical phylogenetics in the three principal northern temperate genera of water striders Limnoporus STÅL 1868, Aquarius SCHELLENBERG 1800 and Gerris FABRICIUS 1794. Most of the discussion is based on com- parison of a recently published combined analysis tree involving four genetic markers and a morpholo- gical data set with older phylogenetic trees primarily based on manual cladistic optimization of mor- phological characters. Key words: DNA-barcodes, Gerrinae, phylogeography, simultaneous analyses. Introduction nally, water striders show great variation in mating strategies, and morphological and Water striders (Hemiptera-Heteroptera, behavioral adaptations to accomplish or Gerromorpha, Gerridae) are familiar inhab- avoid multiple mating (ANDERSEN 1994, itants of aquatic habitats throughout the 1996; ARNQVIST 1997). The striking diver- Worlds temperate, subtropical, and tropical sity in habitat selection, wing polymorphism regions comprising approximately 640 de- and mating strategies – along with the prac- scribed species in 72 genera (ANDERSEN & tically two dimensional habitat, has made WEIR 2004). Most water striders are found water striders popular objects in studies of in freshwater habitats, such as rivers, behavior, ecology and evolution (SPENCE & streams, lakes and ponds, but a few genera ANDERSEN 1994; ROWE et al. -

Coevolution of Male and Female Genital Morphology in Waterfowl Patricia L
Coevolution of Male and Female Genital Morphology in Waterfowl Patricia L. R. Brennan1,2*, Richard O. Prum1, Kevin G. McCracken3, Michael D. Sorenson4, Robert E. Wilson3, Tim R. Birkhead2 1 Department of Ecology and Evolutionary Biology, and Peabody Natural History Museum, Yale University, New Haven, Connecticut, United States of America, 2 Department of Animal and Plant Sciences, University of Sheffield, Western Bank, Sheffield, United Kingdom, 3 Institute of Arctic Biology, Department of Biology and Wildlife, and University of Alaska Museum, University of Alaska Fairbanks, Fairbanks, Alaska, United States of America, 4 Department of Biology, Boston University, Boston, Massachusetts, United States of America Most birds have simple genitalia; males lack external genitalia and females have simple vaginas. However, male waterfowl have a phallus whose length (1.5–.40 cm) and morphological elaborations vary among species and are positively correlated with the frequency of forced extra-pair copulations among waterfowl species. Here we report morphological complexity in female genital morphology in waterfowl and describe variation vaginal morphology that is unprecedented in birds. This variation comprises two anatomical novelties: (i) dead end sacs, and (ii) clockwise coils. These vaginal structures appear to function to exclude the intromission of the counter-clockwise spiralling male phallus without female cooperation. A phylogenetically controlled comparative analysis of 16 waterfowl species shows that the degree of vaginal elaboration is positively correlated with phallus length, demonstrating that female morphological complexity has co-evolved with male phallus length. Intersexual selection is most likely responsible for the observed coevolution, although identifying the specific mechanism is difficult. Our results suggest that females have evolved a cryptic anatomical mechanism of choice in response to forced extra-pair copulations. -
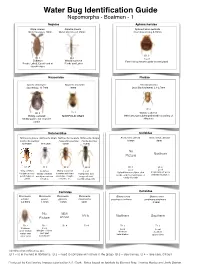
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Mating Changes the Genital Microbiome in Both Sexes of the Common Bedbug
bioRxiv preprint doi: https://doi.org/10.1101/819300; this version posted October 28, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Research article 2 3 4 Mating changes the genital microbiome in both sexes of the common bedbug 5 Cimex lectularius across populations 6 7 8 Running title: Mating-induced changes in bedbug genital microbiomes 9 10 11 Sara Bellinvia1, Paul R. Johnston2, Susan Mbedi3,4, Oliver Otti1 12 13 14 1 Animal Population Ecology, Animal Ecology I, University of Bayreuth, Universitätsstraße 30, 95440 15 Bayreuth, Germany 16 2 Institute for Biology, Free University Berlin, Königin-Luise-Straße 1-3, 14195 Berlin, Germany. 17 3 Museum für Naturkunde - Leibniz-Institute for Evolution and Biodiversity Research, Invalidenstraße 18 43, 10115 Berlin. 19 4 Berlin Center for Genomics in Biodiversity Research (BeGenDiv), Königin-Luise-Straße 1-3, 14195 20 Berlin, Germany. 21 22 23 Corresponding author: Sara Bellinvia 24 Phone: +49921552646, e-mail: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/819300; this version posted October 28, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 25 ABSTRACT 26 Many bacteria live on host surfaces, in cells, and specific organ systems. Although gut microbiomes of 27 many organisms are well-documented, the bacterial communities of reproductive organs, i.e. genital 28 microbiomes, have received little attention. During mating, male and female genitalia interact and 29 copulatory wounds can occur, providing an entrance for sexually transmitted microbes. -

Butterflies of North America
Insects of Western North America 7. Survey of Selected Arthropod Taxa of Fort Sill, Comanche County, Oklahoma. 4. Hexapoda: Selected Coleoptera and Diptera with cumulative list of Arthropoda and additional taxa Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University, Fort Collins, CO 80523-1177 2 Insects of Western North America. 7. Survey of Selected Arthropod Taxa of Fort Sill, Comanche County, Oklahoma. 4. Hexapoda: Selected Coleoptera and Diptera with cumulative list of Arthropoda and additional taxa by Boris C. Kondratieff, Luke Myers, and Whitney S. Cranshaw C.P. Gillette Museum of Arthropod Diversity Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, Colorado 80523 August 22, 2011 Contributions of the C.P. Gillette Museum of Arthropod Diversity. Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523-1177 3 Cover Photo Credits: Whitney S. Cranshaw. Females of the blow fly Cochliomyia macellaria (Fab.) laying eggs on an animal carcass on Fort Sill, Oklahoma. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management, Colorado State University, Fort Collins, Colorado, 80523-1177. Copyrighted 2011 4 Contents EXECUTIVE SUMMARY .............................................................................................................7 SUMMARY AND MANAGEMENT CONSIDERATIONS