The Smaller Groups Polyplacophora, Scaphopoda, and Cephalopoda Alan L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
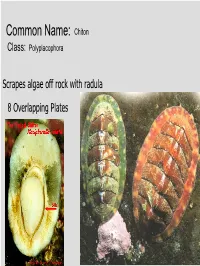
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Enrico SCHWABE Zoologische Staatssammlung Muenchen
. , E. SCHWABE NOVAPEX 6 (4): 89-105, 10 décembre 2005 A catalogue of Récent and fossil chitons (MoUusca: Polyplacophora) Addenda Enrico SCHWABE Zoologische Staatssammlung Muenchen, Muenchhausenstrasse 2 1 D-81247 Muenchen, Germany [email protected] KEYWORDS. MoUusca, Polyplacophora, taxon list, bibliography ABSTRACT. This paper lists species-group names of Récent and fossil Polyplacophora (MoUusca) that were published after 1998 (for the Récent species) and 1987 (for the fossil species). A total of 171 species were since then introduced, of which 123 are attributed to valid fossil taxa and 48 to valid Récent taxa. The authorship and complète références are provided for each species-group name. INTRODUCTION Considerazioni suUa famiglia Leptochitonidae Dali, 1889 (MoUusca: Polyplacophora). III. Le species Taxonomic work is impossible without an overview of terziarie e quatemarie Europee, con note sistematiche the scientific names existing in the particular taxon e filogenetiche. - Atti délia prima Giornata di Studi group. Catalogues generally are a great tool to obtain Malacologici Centra lîaliano di Studi Malacologici such overviews, as they often summarize information (1989): 19-140 (: 79; pi. 26). otherwise hard to gather and master. Type locality: Pezzo, near Villa S. Giovanni (Reggio Of the nearly 2600 taxa introduced on species level Calabria prov.); in material of upper Pleistocene, but within the Polyplacophora, 368 fossils and 914 Récent presumably originated from adjacent deposits of lower species are considered as valid (closing date: Pleistocene of bathyal faciès [Pezzo, presso Villa S. 31/10/2005). Giovanni (RC); in materiale del Pleistocene superiore, In the past, excellent catalogues of species-group ma presumibilmente originato da contigui depositi del names in Polyplacophora were compiled by Kaas & Pleistocene inferiore di faciès batiale]. -

Marine Invertebrate Field Guide
Marine Invertebrate Field Guide Contents ANEMONES ....................................................................................................................................................................................... 2 AGGREGATING ANEMONE (ANTHOPLEURA ELEGANTISSIMA) ............................................................................................................................... 2 BROODING ANEMONE (EPIACTIS PROLIFERA) ................................................................................................................................................... 2 CHRISTMAS ANEMONE (URTICINA CRASSICORNIS) ............................................................................................................................................ 3 PLUMOSE ANEMONE (METRIDIUM SENILE) ..................................................................................................................................................... 3 BARNACLES ....................................................................................................................................................................................... 4 ACORN BARNACLE (BALANUS GLANDULA) ....................................................................................................................................................... 4 HAYSTACK BARNACLE (SEMIBALANUS CARIOSUS) .............................................................................................................................................. 4 CHITONS ........................................................................................................................................................................................... -

Phylum MOLLUSCA Chitons, Bivalves, Sea Snails, Sea Slugs, Octopus, Squid, Tusk Shell
Phylum MOLLUSCA Chitons, bivalves, sea snails, sea slugs, octopus, squid, tusk shell Bruce Marshall, Steve O’Shea with additional input for squid from Neil Bagley, Peter McMillan, Reyn Naylor, Darren Stevens, Di Tracey Phylum Aplacophora In New Zealand, these are worm-like molluscs found in sandy mud. There is no shell. The tiny MOLLUSCA solenogasters have bristle-like spicules over Chitons, bivalves, sea snails, sea almost the whole body, a groove on the underside of the body, and no gills. The more worm-like slugs, octopus, squid, tusk shells caudofoveates have a groove and fewer spicules but have gills. There are 10 species, 8 undescribed. The mollusca is the second most speciose animal Bivalvia phylum in the sea after Arthropoda. The phylum Clams, mussels, oysters, scallops, etc. The shell is name is taken from the Latin (molluscus, soft), in two halves (valves) connected by a ligament and referring to the soft bodies of these creatures, but hinge and anterior and posterior adductor muscles. most species have some kind of protective shell Gills are well-developed and there is no radula. and hence are called shellfish. Some, like sea There are 680 species, 231 undescribed. slugs, have no shell at all. Most molluscs also have a strap-like ribbon of minute teeth — the Scaphopoda radula — inside the mouth, but this characteristic Tusk shells. The body and head are reduced but Molluscan feature is lacking in clams (bivalves) and there is a foot that is used for burrowing in soft some deep-sea finned octopuses. A significant part sediments. The shell is open at both ends, with of the body is muscular, like the adductor muscles the narrow tip just above the sediment surface for and foot of clams and scallops, the head-foot of respiration. -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

Chitons (Mollusca: Polyplacophora) Known from Benthic Monitoring Programs in the Southern California Bight
ISSN 0738-9388 THE FESTIVUS A publication of the San Diego Shell Club Volume XLI Special Issue June 11, 2009 Chitons (Mollusca: Polyplacophora) Known from Benthic Monitoring Programs in the Southern California Bight Timothy D. Stebbins and Douglas J. Eernisse COVER PHOTO Live specimen of Lepidozona sp. C occurring on a piece of metal debris collected off San Diego, southern California at a depth of 90 m. Photo provided courtesy of R. Rowe. Vol. XLI(6): 2009 THE FESTIVUS Page 53 CHITONS (MOLLUSCA: POLYPLACOPHORA) KNOWN FROM BENTHIC MONITORING PROGRAMS IN THE SOUTHERN CALIFORNIA BIGHT TIMOTHY D. STEBBINS 1,* and DOUGLAS J. EERNISSE 2 1 City of San Diego Marine Biology Laboratory, Metropolitan Wastewater Department, San Diego, CA, USA 2 Department of Biological Science, California State University, Fullerton, CA, USA Abstract: About 36 species of chitons possibly occur at depths greater than 30 m along the continental shelf and slope of the Southern California Bight (SCB), although little is known about their distribution or ecology. Nineteen species are reported here based on chitons collected as part of long-term, local benthic monitoring programs or less frequent region-wide surveys of the entire SCB, and these show little overlap with species that occur at depths typically encountered by scuba divers. Most chitons were collected between 30-305 m depths, although records are included for a few from slightly shallower waters. Of the two extant chiton lineages, Lepidopleurida is represented by Leptochitonidae (2 genera, 3 species), while Chitonida is represented by Ischnochitonidae (2 genera, 6-9 species) and Mopaliidae (4 genera, 7 species). -

Chiton (Chiton) Articulatus (MOLLUSCA: POLYPLACOPHORA) DE LA COSTA ROCOSA DE PUERTO ÁNGEL, OAXACA, MÉXICO
INSTITUTO POLITECNICO NACIONAL CENTRO INTERDISCIPLINARIO DE CIENCIAS MARINAS MADURACIÓN GONÁDICA, CICLO REPRODUCTIVO Y TALLA DE MADUREZ SEXUAL DEL QUITÓN Chiton (Chiton) articulatus (MOLLUSCA: POLYPLACOPHORA) DE LA COSTA ROCOSA DE PUERTO ÁNGEL, OAXACA, MÉXICO TESIS QUE PARA OBTENER EL GRADO DE MAESTRÍA EN CIENCIAS EN MANEJO DE RECURSOS MARINOS PRESENTA QUETZALLI YASU ABADIA CHANONA LA PAZ, B.C.S., JULIO 2015 SIP-14 BIS INSTITUTO POLITÉCNICO NACIONAL SECRETARIA DE INVESTIGACIÓN Y POSGRADO ACTA DE REVISIÓN DE TESIS En la Ciudad de La Paz, B.CS,, siendo las i2:Q0 horas del día 18 del mes de Junio del 2015 se reunieron los miembros de la Comisión Revisora de Tesis designada por el Colegio de Profesores de Estudios de Posgrado e Investigación de CICIMAR para examinar la tesis titulada: "MADURACIÓN GONÁDICA, CICLO REPRODUCTIVO Y TALLA DE MADUREZ SEXUAL DEL QUITÓN Chiton (Chkorí) articulatus (Mollusca: Polyplacophora) DE LA COSTA ROCOSA DE PUERTO ÁNGEL, OAXACA, MÉXICO" Presentada por el alumno: ABADÍA CHANONA QUETZALLI YASU Apellido paterno materno nombre(s2 B 1 3 0 8 4 9 Con registro: Aspirante de: MAESTRÍA EN CIENCIAS EN MANEJO DE RECURSOS MARINOS Después de intercambiar opiniones los miembros de la Comisión manifestaron APROBAR LA DEFENSA DELA TESIS, en virtud de que satisface los requisitos señalados por las disposiciones reglamentarias vigentes. &BRIEL MORENO SANCHEZ INSTITUTO POLITÉCNICO NACIONAL SECRETAíRÍA DE INVESTIGACIÓN Y POSGRADO CARTA CESIÓN DE DERECHOS En la Ciudad de La Paz, B.C.S., el día 22 del mes lunio del año 2015 el (la) que suscribe BM. QUETZALLIYASÚABA alumno(a) del Programa de MAESTRÍA EN CIENCIAS EN MANEJO DE RECURSOS MARINOS con número de registro B130849 adscrito al CENTRO INTERDISCIPLINARIO DE CIENCIAS MARINAS manifiesta que es autor (a) intelectual del presente trabajo de tesis, bajo la dirección de: DR. -

A Review of Ethnographic and Historically Recorded Dentaliurn Source Locations
FISHINGFOR IVORYWORMS: A REVIEWOF ETHNOGRAPHICAND HISTORICALLY RECORDEDDENTALIUM SOURCE LOCATIONS Andrew John Barton B.A., Simon Fraser University, 1979 THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS IN THE DEPARTMENT OF ARCHAEOLOGY Q Andrew John Barton 1994 SIMON FRASER UNIVERSITY Burnaby October, 1994 All rights reserved. This work may not be reproduced in whole or in part, by photocopy or other means without permission of the author. Name: Andrew John Barton Degree: Master of Arts (Archaeology) Title of Thesis: Fishing for Ivory Worms: A Review of Ethnographic and Historically Recorded Dentaliurn Source Locations Examining Committee: Chairperson: Jack D. Nance - -, David V. Burley Senior Supervisor Associate Professor Richard Inglis External Examiner Department of Aboriginal Affairs Government of British Columbia PARTIAL COPYRIGHT LICENSE I hereby grant to Simon Fraser University the right to lend my thesis or dissertation (the title of which is shown below) to users of the Simon Fraser University Library, and to make partial or single copies only for such users or in response to a request from the library of any other university, or other educational institution, on its own behalf or for one of its users. I further agree that permission for multiple copying of this thesis for scholarly purposes may be granted by me or the Dean of Graduate Studies. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Title of ThesisIDissertation: Fishing for Ivory Worms: A Review of Ethnographic and Historically Recorded Dentalium Source Locations Author: Andrew John Barton Name October 14, 1994 Date This study reviews and examines historic and ethnographic written documents that identify locations where Dentaliurn shells were procured by west coast Native North Americans. -

Mollusca) Found Along the Brazilian Coast, with Two New Synonymies in the Genus Gadila Gray, 1847
Biota Neotrop., vol. 13, no. 2 A commented list of Scaphopoda (Mollusca) found along the Brazilian coast, with two new synonymies in the genus Gadila Gray, 1847 Leonardo Santos de Souza1,2, Isabella Campos Vieira Araújo1 & Carlos Henrique Soares Caetano1 1Departamento de Zoologia, Instituto de Biociências, Universidade Federal do Estado do Rio de Janeiro – UNIRIO, Av. Pasteur, 458, Urca, CEP 22290-240, Rio de Janeiro, RJ, Brasil 2Corresponding author: Leonardo Santos de Souza, e-mail: [email protected] SOUZA, L.S., ARAÚJO, I.C.V. & CAETANO, C.H.S. A commented list of Scaphopoda (Mollusca) found along the Brazilian coast, with two new synonymies in the genus Gadila Gray, 1847. Biota Neotrop. (13)2: http://www.biotaneotropica.org.br/v13n2/en/abstract?inventory+bn03213022013 Abstract: This review aims to present an updated checklist of scaphopods, based mainly on literature database. There is a total of 40 species (six families) for Brazil, including information about the distribution and bathymetric range of each taxon. We propose two synonyms with the aid of morphometry of the shell, for the genus Gadila: G. longa as junior synonym of G. elongata and G. robusta as junior synonym of G. pandionis. Keywords: scaphopods, morphometry, synonyms, distribution, bathymetry. SOUZA, L.S., ARAÚJO, I.C.V. & CAETANO, C.H.S. Lista comentada dos Scaphopoda (Mollusca) encontrados ao longo da costa Brasileira, com duas novas sinonímias no gênero Gadila Gray, 1847. Biota Neotrop. 13(2): http://www.biotaneotropica.org.br/v13n2/pt/abstract?inventory+bn0321302201 Resumo: Uma lista atualizada dos escafópodes da costa brasileira pertencentes a seis famílias é apresentada baseada principalmente em dados da literatura. -

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean. -

Biodiversidad Barra.Pdf
BIODIVERSIDAD DE BARRA DE POTOSÍ, GUERRERO, MÉXICO. Hacia una interacción entre conservación y turismo barra_potosi(2a_ed).indd 1 06/05/20 9:26 barra_potosi(2a_ed).indd 2 06/05/20 9:26 BIODIVERSIDAD DE BARRA DE POTOSÍ, GUERRERO, MÉXICO. Hacia una interacción entre conservación y turismo Alejandro Meléndez Herrada | Aurora Chimal Hernández Ana Luisa Figueroa Fernández | Falco Manuel García González Antonio Isain Contreras Rodríguez | Elisa Vázquez Suaste UNIVERSIDAD AUTÓNOMA METROPOLITANA Casa abierta al tiempo UNIVERSIDAD AUTÓNOMA METROPOLITANA UnidadRector Xochimilco General Dr. Eduardo Abel Peñalosa Castro Secretario General Dr. José Antonio de los Reyes Heredia UNIVERSIDAD AUTÓNOMA METROPOLITANA-XOCHIMILCO Rector Dr. Fernando de León González Secretaria Dra. Claudia Mónica Salazar Villava DIVISIÓN DE CIENCIAS BIOLÓGICAS Y DE LA SALUD barra_potosi(2a_ed).indd 3 06/05/20 9:26 Directora Mtra. María Elena Contreras Garfias Secretario Académico Dr. Luis Amado Ayala Pérez Responsable del Programa Editorial Mtra. Zyanya Patricia Ruiz Chapoy Comité Editorial Dr. Edgar Carlos Jarillo Soto Mtro. Felipe Mendoza Pérez Dr. Jorge Esteban Miranda Calderón Biól. José Alfredo Arévalo Ramírez Dr. José Antonio Herrera Barragán Dr. José Arturo Granados Cosme Dr. José Francisco Cervantes Mayagoitia Dra. Patricia Castilla Hernández “Biodiversidad de Barra de Potosí, Guerrero, México. Hacia una interacción entre conservación y turismo” Primera edición: 2019 ISBN: 978-607-28-1628-2 Fotos de portada: Alejandro Meléndez y Falco M. García D.R. © UNIVERSIDAD AUTÓNOMA METROPOLITANA Unidad Xochimilco Calzada Del Hueso 1100 Col. Villa Quietud, Alcaldía Coyoacán C.P. 04960, Ciudad de México, Tel.: 5483 7000 ext. 3783 Impreso y hecho en México UNIVERSIDAD AUTÓNOMA METROPOLITANA Casa abierta al tiempo UNIVERSIDAD AUTÓNOMA METROPOLITANA UnidadRector Xochimilco General Dr. -

Marine Shells of the Western Coast of Flordia
wm :iii! mm ilili ! Sfixing cHdL J^oad .Sandivicl'i, j\{ai.i.ach.u±£.tti. icuxucm \^*^£ FRONTISPIECE Photo by Ruth Bernhard Spondylus americanus Hermann MARINE SHELLS f>4 OF THE WESTERN COAST OF FLORIDA By LOUISE M. PERRY AND JEANNE S. SCHWENGEL With Revisions and Additions to Louise M. Perry's Marine Shells of the Southwest Coast of Florida Illustrations by W. Hammersley Southwick, Axel A. Olsson, and Frank White March, 1955 PALEONTOLOGICAL RESEARCH INSTITUTION ITHACA, NEW YORK U. S. A. MARINE SHELLS OF THE SOUTHWEST COAST OF FLORIDA printed as Bulletins of American Paleontology, vol. 26, No. 95 First printing, 1940 Second printing, 1942 Copyright, 1955, by Paleontological Research Institution Library of Congress Catalog Card Number: 5-^-12005 Printed in the United States of America // is perhaps a more fortunate destiny to have a taste for collecting shells than to be born a millionaire. Robert Louis Stevenson imeters 50 lllllllllllllllllllllllllllll II II III nil 2 Inches CONTENTS Page Preface by reviser 7 Foreword by Wm. J. Clench 9 Introduction 11 Generalia 13 Collection and preparation of specimens 17 Systematic descriptions 24 Class Amphineura :. 24 Class Pelecypoda 27 Class Scaphopoda 97 Class Gasteropoda 101 Plates 199 Index 311 PREFACE BY THE REVISER It has been a privilege to revise Louise M. Perry's fine book on "Marine Shells of Southwest Florida", to include her studies on eggs and larvae of mollusks; and to add descriptions and illustra- tions of several newly discovered shells thus making it a more com- prehensive study of the molluscan life of western Florida. The work that I have done is only a small return to Dr.