A Case Study Using the Triassic Montney Formation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alberta Basin. See Western Canada Foreland Basin. Alexander Terrane
— ♦ — Index — ♦ — Alberta basin. See Western Canada foreland basin. Cardium Formation, 33, 35, 201, 202, 265, 302 Alexander terrane, 85 Cardium reservoir unit, 177, 178 Andean-type continental margin magmatic arc, Cascade basin, 273, 274 subduction along, 10 Cascade terrane, 85 Arctic Alaska plate, 376 Cassiar terrane, 85 Arctic National Wildlife Refuge (ANWR), 363 Charlie Lake Formation, 296 Arkoma basin, 436, 437, 443 Chinook Member, 36 Asmari reservoir, 331-332 Chugach terrane, 85, 116 Chungo Member, 33, 36 Bangestan/Qamchuqa reservoir, 332-333 Clearwater Formation, 27 Barrow arch, 375 Coastal belt terrane, 116, 118 Basal Colorado Sand reservoir unit, 170, 171 Coast plutonic complex, 11, 83 Bearpaw sandstones, 236 Colony Formation, 28 Beattie Peaks Formation, 18 Colorado Assemblage, 192,196-197, 199-201, 210-212, Belloy Formation, 297 224-226 Belly River Formation, 38, 117, 272 Colorado Group, 281-286, 300-303 Belly River reservoir unit, 181, 182 Colorado Group oils, 300 Belly River sandstones, 236 Colorado Shale, 284, 286 Belt Supergroup, 275 Crescent terrane, 116 Black Warrior basin, 431, 435, 436 Cummings Member, 24 Bluesky Formation, 22, 25 Cypress Hills/Hand Hills Assemblage, 192-193, 196-197, Boulder Batholith, 238 202-203 Bow Island Formation, 31, 32 Cypress Hills Formation, 42 Bowser basin, 85 Brazeau Formation, 39 Dakota sandstone, 414 Bridge River terrane, 85 Deep basin, Rock-Eval pyrolysis and TOC data, 283 Brookian sequence, 369-370, 370-374 Deformed belt, 84, 268-269 Dezful embayment. See Zagros foreland basin. Cache Creek -
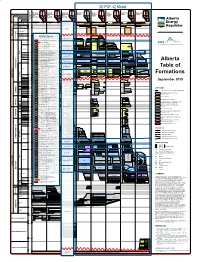
Alberta Table of Formations
3D PGF v2 Model Fort Northern Central Southern Southern West- East- Northwest Northeast McMurray Grande ERATHEM / ERA Mountains Mountains Mountains Plains Central Central Plains Prairie Plains and Edmonton and Edmonton and Edmonton Edmonton Plains Edson Edmonton Plains Edmonton Edmonton Edmonton SYSTEM / PERIOD Foothills Jasper Foothills Foothills OF YEARS Banff SERIES / EPOCH Calgary AGE IN MILLIONS Blairmore Medicine STAGE / AGE Hat GC MC GC MC GC MC Laurentide Laurentide B Laurentide B B Laurentide Cordilleran Laurentide Cordilleran Laurentide Laurentide Cordilleran Cordilleran Laurentide BL BL QUATERNARY PLEISTOCENE Cordilleran EMPRESS EMPRESS EMPRESS EMPRESS EMPRESS PLIOCENE 2.6 5.3 DEL BONITA ARROWWOOD HAND HILLS WHITECOURT MOUNTAIN HALVERSON RIDGE PELICAN MOUNTAIN 7 NEOGENE MIOCENE 23 zone 1 OLIGOCENE CYPRESS HILLS OBED MOUNTAIN SWAN HILLS 34 EOCENE CENOZOIC 56 DALEHURST PALEOGENE PASKAPOOModel ZonesPASKAPOO PASKAPOO LACOMBE PALEOCENE HAYNES zonesPORCUPINE 2-3 HILLS PORCUPINE HILLS UPPER UPPER COALSPUR COALSPUR UPPER RAVENSCRAG UPPER 66 LOWER WILLOW CREEK WILLOW CK SCOLLARD SCOLLARD 01ENTRANCEsediment CONGLOMERATE above bedrock (surficial deposits) LOWER LOWER FRENCHMAN LOWER BATTLE BATTLE BATTLE WHITEMUD WHITEMUD MAASTRICHTIAN Bedrock topography CARBON ST. MARY RIVER ST. MARY RIVER HORSESHOE TOLMAN MORRIN UPPER UPPER EASTEND CANYON HORSESHOE 02 Paskapoo and Porcupine Hills formations HORSETHIEF CANYON 72 DRUMHELLER BLOOD RESERVE BLOOD RESERVE EDMONTON UPPER SAUNDERS SAUNDERS WAPITI / BRAZEAU STRATHMORE BRAZEAUScollard, Willow -

The Universw of Calgary a Thesis Submitted to The
THE UNIVERSW OF CALGARY Isotopic and Compositional Characterization of Natural Gases in the Lower and Middle Triassic Montney, Halfway, and Doig Formations, Alberta Basin Steven Desrocher A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STCTDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE DEPARTMENT OF GEOLOGY AND GEOPHY SICS CALGARY, ALBERTA DECEMBER, 1997 0 Steven Desrocher 1997 National Library Bibliothéque natiode u*m of Canada du Canada Acquisitions and Acquisitions et Bibliographie Services services bibliographiques 395 Wellington Street 395. nie Wellington OttawaON K1AW Ottawa ON KIA ON4 Canada canada The author has granted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive permettant à la National Lîbrary of Canada to Bibliothèque nationale du Canada de reproduce, loan, distribute or seil reproduire, prêter, distriiuer ou copies of this thesis in microfom, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/nlm, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fiom it Ni la thèse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation, ABSTRACT Natural gases in the Montney, Doig, and Halfway formations are charactenzed by variations in the proportion of methane to higher alkanes, affecting caionfic and commercial values. Stable isotope and compositional analyses suggest that wet gases in the central study am are produced via active thermogenic gas generation, with gases sourced in the Doig and Montney formations. -

Fluid Compartmentalization of Devonian and Mississippian Dolostones, Western Canada Sedimentary Basin: Evidence from Fracture Mineralization
University of Windsor Scholarship at UWindsor Electronic Theses and Dissertations Theses, Dissertations, and Major Papers 2016 Fluid Compartmentalization of Devonian and Mississippian Dolostones, Western Canada Sedimentary Basin: Evidence from Fracture Mineralization Carole Mrad University of Windsor Follow this and additional works at: https://scholar.uwindsor.ca/etd Recommended Citation Mrad, Carole, "Fluid Compartmentalization of Devonian and Mississippian Dolostones, Western Canada Sedimentary Basin: Evidence from Fracture Mineralization" (2016). Electronic Theses and Dissertations. 5752. https://scholar.uwindsor.ca/etd/5752 This online database contains the full-text of PhD dissertations and Masters’ theses of University of Windsor students from 1954 forward. These documents are made available for personal study and research purposes only, in accordance with the Canadian Copyright Act and the Creative Commons license—CC BY-NC-ND (Attribution, Non-Commercial, No Derivative Works). Under this license, works must always be attributed to the copyright holder (original author), cannot be used for any commercial purposes, and may not be altered. Any other use would require the permission of the copyright holder. Students may inquire about withdrawing their dissertation and/or thesis from this database. For additional inquiries, please contact the repository administrator via email ([email protected]) or by telephone at 519-253-3000ext. 3208. Fluid Compartmentalization of Devonian and Mississippian Dolostones, Western Canada Sedimentary -

Hydrocarbon-Generation Kinetics of the Doig Formation, Northeastern British Columbia and West-Central Alberta
Hydrocarbon-Generation Kinetics of the Doig Formation, Northeastern British Columbia and West-Central Alberta P.L. Silva1, The University of British Columbia, Vancouver, British Columbia, [email protected] R.M. Bustin, The University of British Columbia, Vancouver, British Columbia Silva, P.L. and Bustin, R.M. (2020): Hydrocarbon-generation kinetics of the Doig Formation, northeastern British Columbia and west- central Alberta; in Geoscience BC Summary of Activities 2019: Energy and Water, Geoscience BC, Report 2020-02, p. 65–76. Introduction Formation petroleum system and the results of the reaction kinetics presented here provide the inputs required to pro- Reliable petroleum-system models require kinetic parame- ceed with the subsequent steps in modelling thermogenic ters of the reaction induced by thermal decomposition of hydrocarbon generation across the basin. kerogen into hydrocarbons to be calibrated to the source rock (Peters et al., 2018). Formation-specific kinetic pa- rameters for the Doig Formation are not in the public do- Geological Framework main; therefore, any previous attempt to model the hydro- The Doig was deposited in the Middle Triassic, between the carbon generation history would have required kinetic Anisian and Ladinian, and is part of the Diaber Group along analyses to be undertaken or reliance on analogue data, with the underlying Montney Formation (Figure 1). The both of which may produce erroneous results. sedimentation in the Triassic of the WCSB is marked by a The Lower to Middle Triassic Doig Formation of the West- transition from carbonate-dominated intracratonic and pas- ern Canada Sedimentary Basin (WCSB) extends continu- sive-margin conditions, predominant during the Paleozoic, ously across northeastern British Columbia (BC) and west- to a siliciclastic-dominated, active embryonic foreland ba- central Alberta. -

AG412&3 D05 FINAL.Indd
Atlantic Geology 187 Atlantic Universities Geological Conference 2005 ABSTRACTS October 27 – 29, 2005 Conference hosted by: The Alexander Murray Geology Club Memorial University of Newfoundland St. John’s, Newfoundland Again this year, abstracts from the annual Atlantic Universities Geological Conference (AUGC) are pub- lished in Atlantic Geology. This provides a permanent record of the abstracts, and also focuses attention on the excellent quality of the presentations and posters and the interesting and varied geoscience that they cover. The abstracts are published with financial assistance from APICS. THE EDITORS Atlantic Geology 41, 186–194 (2005) 0843-5561|05|020186–08$2.20|o 188 AUGC Abstracts – 2005 Atlantic Geology 189 Coupling of salt dynamics and sedimentary basin detailed cross sections throughout the area, structure maps and evolution on passive margins: implications for isopach maps, along with a variety of other maps. A study of the offshore Nova Scotia hydrocarbon exploration capping sands and the underlying Doig shales would improve the technical understanding of the area surrounding the Doig Sheila Ballantyne reservoir by means of core analysis and detailed photographs Department of Earth Sciences, Dalhousie University, of contacts and both depositional and biological sedimentary Halifax, NS B3H 3J5 features. Ichnology may play a role in helping to create a depo- sitional model for the capping sands of the Doig shales and The high economic risk in hydrocarbon exploration on the the related Doig thick sand bodies. This would benefit future Scotian Slope, offshore Nova Scotia, is caused in part by the models proposed for the ATSBs. complex salt structures that have developed in this area. -

Factsheet 3Col V
National and Global Petroleum Assessment Assessment of Continuous Gas Resources in the Montney and Doig Formations, Alberta Basin Province, Canada, 2018 Using a geology-based assessment methodology, the U.S. Geological Survey estimated undiscovered, technically recoverable mean resources of 47.6 trillion cubic feet of gas and 2.2 billion barrels of natural gas liquids in the Montney and Doig Formations of the Alberta Basin Province in Canada. Introduction Shale Gas AU was defined to encompass areas of organic-rich shale The U.S. Geological Survey (USGS) quantitatively assessed within the overpressured gas-generation window. the potential for undiscovered, technically recoverable continuous Assessment input data are summarized in table 1. Input data (unconventional) gas resources in the Triassic Montney and Doig from wells within drainage areas in the upper part of the Montney Formations of the Alberta Basin Province of Canada (fig. 1). In this are based mainly on Schmitz and others (2014) and Kwan (2015). study, the upper Montney Formation siltstones were assessed as a Drainage areas (for shales of the Doig Formation), success ratios, tight-gas accumulation (Chalmers and Bustin, 2012), and the Doig and estimated ultimate recoveries of wells are taken from geologic Formation phosphatic shales were assessed as a potential shale-gas analogs in the United States. accumulation (Chalmers and others, 2012). Total Petroleum Systems and Assessment Units 126° W 122° W 118° W 114° W The USGS defined an Upper Montney Total Petroleum System (TPS) and a Doig TPS. The upper part of the Montney Formation consists of organic-bearing siltstones that represent distal shelf, slope, and basinal turbidite deposits. -

The Ultimate Potential for Unconventional Petroleum from The
Permission to Reproduce Materials may be reproduced for personal, educational and/or non-profit activities, in part or in whole and by any means, without charge or further permission from the National Energy Board, the British Columbia Oil and Gas Commission, the Alberta Energy Regulator, or the British Columbia Ministry of Natural Gas Development, provided that due diligence is exercised in ensuring the accuracy of the information reproduced; that the National Energy Board, the British Columbia Oil and Gas Commission, the Alberta Energy Regulator, and the British Columbia Ministry of Natural Gas Development are identified as the source institutions; and that the reproduction is not represented as an official version of the information reproduced, nor as having been made in affiliation with or with the endorsement of the National Energy Board, the British Columbia Oil and Gas Commission, the Alberta Energy Regulator, or the British Columbia Ministry of Natural Gas Development. For permission to reproduce the information in this publication for commercial redistribution, please e-mail the National Energy Board at info@neb- one.gc.ca, the British Columbia Oil and Gas Commission at [email protected] the Alberta Energy Regulator at [email protected], or the British Columbia Ministry of Natural Gas Development at [email protected]. Autorisation de reproduction Le contenu de cette publication peut être reproduit à des fins personnelles, éducatives et/ou sans but lucratif, en tout ou en partie et par quelque moyen que ce soit, sans frais -

Back Matter (PDF)
Index adsorption 15-16 Caithness Flags 168 adsorption chromatography 152, 153 Canada see Nova Scotia Basin also Western Canada Age anticline 243 Sedimentary Basin Alabama see Mexican Gulf Coast carbon dioxide and migration 156 Alberta 218 carbon isotope ratios 193, 195 Alpine foreland carbon mass balance 11 maturation Carboniferous temperature 240-1 reservoirs 35, 41, 42, 218 timing 238-40 source rocks 210 regional setting 227-9 Cardium Formation 124, 209, 216 regional studies cataclasis v. diffusive mass transfer 256 Clergeon anticline 232-5 Central Graben (North Sea) see also Sub-Alpine Chains compaction studies 70-2, 76-9 stratigraphy 230-1 migration studies 52, 175, 177 structure 229 Charlie Lake Formation 209, 210 subsidence history 231-2 Chinchaga-Contact Rapids Formation 212 thrust timing 231 chromatography alteration v. degradation 197 fractionation 144, 145 Alwyn kitchen 91 process applications 152-4 Andrade formula 107 gas-liquid 156-7 Annecy 227, 241,246 gas-solid 156 aqueous fractionation 200, 201-2 liquid-liquid 155-6 aquifer properties 128, 130 liquid-solid 154-5 Arbuckle Formation 201 quantification 157-9 Are Formation 273 results 198 Arkansas 52 techniques 151-2 see also Mexican Gulf Coast theory 150-1 asphalt 227, 241,246 clay minerals Athabasca tar sands 208, 216 adsorption 15-16 Australia case study 24-7 permeability 12-13 Clergeon anticline 229, 232 burial history 232-4 Bakken Shale Formation 51-2 thermal history 234-5 Balder field 178 coal Baider Sandstone 183 oleophilicity 16 Banff Group 209 source potential 23-4 -

Induced Seismicity Study in the Kiskatinaw Seismic Monitoring and Mitigation Area, British Columbia for the BC Oil and Gas Commission
Induced Seismicity Study in the Kiskatinaw Seismic Monitoring and Mitigation Area, British Columbia for the BC Oil and Gas Commission Amy D. Fox, Ph.D., P. Geo. and Neil D. Watson, P. Geol. Final Report June 5, 2019 Disclaimer All work performed for this project and information provided in this report is of the highest technical quality possible given data limitations and uncertainties at the time the work was completed. Enlighten Geoscience Ltd. cannot guarantee the accuracy of the material included in this report (and corresponding presentation) and bears no responsibility for the use of the material. This report was written as a preliminary contribution to understanding issues surrounding induced seismicity in the Kiskatinaw Seismic Monitoring and Mitigation Area. The ability to predict induced seismicity or infer economic valuations is explicitly not meant to be implied from this report. Enlighten Geoscience Ltd. APEGA Permit P13351 Member #159887 June 5, 2019 © 2019 Enlighten Geoscience Ltd. 2 Executive Summary Induced seismicity has been a growing concern throughout North America and around the world as increasing numbers of seismic events have been occurring in historically quiet tectonic areas and appear to be related to localized oil and gas development activity. On November 29, 2018 hydraulic fracture operations were suspended after a series of three seismic events ranging from 3.4 to 4.5 magnitude were recorded in the Kiskatinaw Seismic Monitoring and Mitigation Area (KSMMA) in northeast British Columbia, Canada. The goal of this study was to perform the first phase of a larger investigation designed to identify key or common factors coincident with induced seismicity and/or to delineate areas of higher induced seismicity likelihood within the KSMMA. -

Alberta T2L 2A7
GEOLOGICAL SURVEY OF CANADA COMMISSION GEOLOGIQUE DU CANADA Podruski, J.A., Barc Canadz , I GEOLOGICAL SURVEY OF CANADA PAPER 87-26 CONVENTIONAL OIL RESOURCES OF WESTERN CANADA (LIGHT AND MEDIUM) PART I: RESOURCE ENDOWMENT Podruski, J.A., Barclay, J.E., Hamblin, A.P., Lee, P.J., Osadetz, K.G., Procter, R.M., and Taylor, G.C. PART II: ECONOMIC ANALYSIS Conn, R.F., and Christie, J.A. @Minister of Supply and Services Canada 1987 Available in Canada through authorized book store agents and other book stores or by mail from Canadian Government Publishing Centre Supply and Services Canada Hull, Quebec, Canada KIA OS9 and from Geological Survey of Canada 601 Booth Street Ottawa, Canada KIA OE8 and Institute of Sedimentary and Petroleum Geology Geological Survey of Canada 3303 - 33rd Street, N.W. Calgary, Alberta T2L 2A7 A deposit copy of this publication is also available for reference in public libraries across Canada Cat. No. M44-87126E Canada: $10.00 ISBN 0-660-1 2650-8 Other Countries: $1 2.00 Price subject to change without notice Scientific Editor P. Fitzgerald-Moore Production manager L. Mac Lachlan Layout and artwork supervised by I. W. Thomson W. P. Vermette Typesetting supervised by P. L. Greener Cartography and Word Processing units Institute of Sedimentary and Petroleum Geology Authors' addresses Podruski, /.A,, Barclay, j.E., Hamblin, A.P., Lee, P.]., Osadetz, K.C., Procter, R.M., and Taylor, C.C. Institute of Sedimentary and Petroleum Geology 3303 - 33rd Street, N.W., Calgary, Alberta T2L 2A7 Conn, R.F., and Christie, ).A. Petroleum Resources Branch Energy, Mines and Resources 580 Booth Street, Ottawa, Ontario KIA 0E4 Cette publication est aussi disponible en francais PREFACE The major oil discovery at Leduc in 1947 led to the develop La decouverte majeure de petrole faite A Leduc en 1947 a men6 h I'essor ment of a vital Canadian petroleum industry. -
Summary of Field Activities in the Western Liard Basin, British Columbia Filippo Ferri, Margot Mcmechan, Tiffani Fraser, Kathryn Fiess, Leanne Pyle, Fabrice Cordey
Summary of field activities in the western Liard Basin, British Columbia Filippo Ferri, Margot Mcmechan, Tiffani Fraser, Kathryn Fiess, Leanne Pyle, Fabrice Cordey To cite this version: Filippo Ferri, Margot Mcmechan, Tiffani Fraser, Kathryn Fiess, Leanne Pyle, et al.. Summary offield activities in the western Liard Basin, British Columbia. Geoscience Reports 2013, British Columbia Ministry of Natural Gas Development, pp.13-32, 2013, Geoscience Reports 2013. hal-03274965 HAL Id: hal-03274965 https://hal.archives-ouvertes.fr/hal-03274965 Submitted on 2 Jul 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. SUMMARY OF FIELD ACTIVITIES IN THE WESTERN LIARD BASIN, BRITISH COLUMBIA Filippo Ferri1, Margot McMechan2, Tiffani Fraser3, Kathryn Fiess4, Leanne Pyle5 and Fabrice Cordey6 ABSTRACT The second and final year of a regional bedrock mapping program within the Toad River map area (NTS 094N) was completed in 2012. The program will result in three– 100 000 scale maps of the northwest, northeast and southeast quadrants of 094N and with four 1:50 000 scale maps covering the southwest quadrant. Surface samples were also collected for Rock Eval™, reflective light thermal maturity and apatite fission-track analysis.