Cooperative National Park Resources Studies Unit Univeristy of Hawai'i at Manoa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kathryn E. Perez, Ph.D. Department of Biology, University of Texas Rio Grande Valley 1201 W
2019 CV Kathryn E. Perez, Ph.D. Department of Biology, University of Texas Rio Grande Valley 1201 W. University Dr., Edinburg, TX 78539 Phone: 956-665-7145 Email: [email protected] URL: http://northamericanlandsnails.org/ Education 2005 Ph.D. Biological Sciences, University of Alabama. 2001 M.S. Biology, Angelo State University. 1998 B.S. Biology, Angelo State University. Professional Experience Assistant Professor, University of Texas Rio Grande Valley, 2014-present. Assistant Professor, University of Texas Pan-American/University of Texas Rio Grande Valley, 2014-2015. Undergraduate Program Coordinator, Biology, University of Texas Rio Grande Valley, 2017-present. Associate Professor, University of Wisconsin at La Crosse, 2012-2014. Assistant Professor, University of Wisconsin at La Crosse, 2008-2012. Member, Assessment of Competence in Experimental Design in Biology (ACED-Bio) Research Coordination Network 2014-2019. Associate, UWL Institute for Latina/o and Latin American Studies. 2008-2014. Research Associate, Section of Mollusks, Carnegie Museum of Natural History, 2005-present. Postdoctoral Fellow, Seeding Postdoctoral Innovators in Research and Education (NIH-SPIRE), 2005-2008. University of North Carolina at Chapel Hill. Visiting Research Scholar, Duke University, Durham NC, Postdoctoral Research with Dr. Cliff Cunningham, 2005-2008. Visiting Assistant Professor, North Carolina Central University, Durham NC, 2007. Graduate Fellow, Integrative Graduate Education and Research Traineeship (NSF-IGERT) program in the Freshwater Sciences, -

Population Structure in the Endemic Hawaiian Amber Snail Succinea Caduca (Mighels, 1845)
Molecular Ecology (2007) 16, 2422–2435 doi: 10.1111/j.1365-294X.2007.03246.x ABlackwell Publishing geographic Ltd mosaic of passive dispersal: population structure in the endemic Hawaiian amber snail Succinea caduca (Mighels, 1845) BRENDEN S. HOLLAND and ROBERT H. COWIE Center for Conservation Research and Training, Pacific Biosciences Research Center, University of Hawaii, 3050 Maile Way, Gilmore 408, Honolulu, Hawaii 96822, USA Abstract We used 276 cytochrome c oxidase subunit I (COI, 645 bp) and a subset of 84 16S large ribosomal subunit (16S, 451 bp) sequences to evaluate geographic patterns of genetic vari- ation in 24 populations of the endemic Hawaiian land snail Succinea caduca spanning its range on six islands. Haplotype networks, gene tree topologies, pairwise molecular divergence and FST matrices suggest substantial geographic genetic structuring and complex dispersal patterns. Low nucleotide diversity and low pairwise molecular divergence values within populations coupled with higher between population values suggest multiple founder events. High overall haplotype diversity suggests diversification involving rare interpopulation dispersal, fragmentation by historical lava flows and variation in habitat structure. Within-island rather than between-island population comparisons accounted for the majority of molecular variance. Although 98% of 153 COI haplotypes were private by population, a Mantel test showed no evidence for isolation by distance. Mismatch dis- tributions and population partitioning patterns suggest that genetic fragmentation has been driven by punctuated, passive dispersal of groups of closely related haplotypes that subsequently expanded and persisted in isolation for long periods (average > 2 million years ago), and that Pleistocene island connections may have been important in enhancing gene flow. -

Fauna of New Zealand Ko Te Aitanga Pepeke O Aotearoa
aua o ew eaa Ko te Aiaga eeke o Aoeaoa IEEAE SYSEMAICS AISOY GOU EESEAIES O ACAE ESEAC ema acae eseac ico Agicuue & Sciece Cee P O o 9 ico ew eaa K Cosy a M-C aiièe acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa EESEAIE O UIESIIES M Emeso eame o Eomoogy & Aima Ecoogy PO o ico Uiesiy ew eaa EESEAIE O MUSEUMS M ama aua Eiome eame Museum o ew eaa e aa ogaewa O o 7 Weigo ew eaa EESEAIE O OESEAS ISIUIOS awece CSIO iisio o Eomoogy GO o 17 Caea Ciy AC 1 Ausaia SEIES EIO AUA O EW EAA M C ua (ecease ue 199 acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa Fauna of New Zealand Ko te Aitanga Pepeke o Aotearoa Number / Nama 38 Naturalised terrestrial Stylommatophora (Mousca Gasooa Gay M ake acae eseac iae ag 317 amio ew eaa 4 Maaaki Whenua Ρ Ε S S ico Caeuy ew eaa 1999 Coyig © acae eseac ew eaa 1999 o a o is wok coee y coyig may e eouce o coie i ay om o y ay meas (gaic eecoic o mecaica icuig oocoyig ecoig aig iomaio eiea sysems o oewise wiou e wie emissio o e uise Caaoguig i uicaio AKE G Μ (Gay Micae 195— auase eesia Syommaooa (Mousca Gasooa / G Μ ake — ico Caeuy Maaaki Weua ess 1999 (aua o ew eaa ISS 111-533 ; o 3 IS -7-93-5 I ie 11 Seies UC 593(931 eae o uIicaio y e seies eio (a comee y eo Cosy usig comue-ase e ocessig ayou scaig a iig a acae eseac M Ae eseac Cee iae ag 917 Aucka ew eaa Māoi summay e y aco uaau Cosuas Weigo uise y Maaaki Weua ess acae eseac O o ico Caeuy Wesie //wwwmwessco/ ie y G i Weigo o coe eoceas eicuaum (ue a eigo oaa (owe (IIusao G M ake oucio o e coou Iaes was ue y e ew eaIa oey oa ue oeies eseac -

Evolutionary History of a Vanishing Radiation
Lee et al. BMC Evolutionary Biology 2014, 14:202 http://www.biomedcentral.com/1471-2148/14/202 RESEARCH ARTICLE Open Access Evolutionary history of a vanishing radiation: isolation-dependent persistence and diversification in Pacific Island partulid tree snails Taehwan Lee1, Jingchun Li1, Celia KC Churchill2 and Diarmaid Ó Foighil1* Abstract Background: Partulid tree snails are endemic to Pacific high islands and have experienced extraordinary rates of extinction in recent decades. Although they collectively range across a 10,000 km swath of Oceania, half of the family’s total species diversity is endemic to a single Eastern Pacific hot spot archipelago (the Society Islands) and all three partulid genera display highly distinctive distributions. Our goal was to investigate broad scale (range wide) and fine scale (within‐Society Islands) molecular phylogenetic relationships of the two widespread genera, Partula and Samoana. What can such data tell us regarding the genesis of such divergent generic distribution patterns, and nominal species diversity levels across Oceania? Results: Museum, captive (zoo) and contemporary field specimens enabled us to genotype 54 of the ~120 recognized species, including many extinct or extirpated taxa, from 14 archipelagoes. The genera Partula and Samoana are products of very distinct diversification processes. Originating at the western edge of the familial range, the derived genus Samoana is a relatively recent arrival in the far eastern archipelagoes (Society, Austral, Marquesas) where it exhibits a stepping‐stone phylogenetic pattern and has proven adept at both intra‐and inter‐ archipelago colonization. The pronounced east–west geographic disjunction exhibited by the genus Partula stems from a much older long-distance dispersal event and its high taxonomic diversity in the Society Islands is a product of a long history of within‐archipelago diversification. -

BULLETIN of the FLORIDA STATE MUSEUM Biological Sciences
BULLETIN of the FLORIDA STATE MUSEUM Biological Sciences VOLUME 29 1983 NUMBER 3 NON-MARINE MOLLUSKS OF BORNEO II PULMONATA: PUPILLIDAE, CLAUSILIIDAE III PROSOBRANCHIA: HYDROCENIDAE, HELICINIDAE FRED G. THOMPSON AND S. PETER DANCE UNIVERSITY OF FLORIDA GAINESVILLE Numbers of the BULLETIN OF THE FLORIDA STATE MUSEUM, BIOLOGICAL SCIENCES, are published at irregular intervals. Volumes contain about 300 pages and are not necessarily completed in any one calendar year. OLIVER L. AUSTIN, JR., Editor RHODA J . BRYANT, Managing Editor Consultants for this issue: JOHN B. BURCH WILLIAM L. PRATT Communications concerning purchase or exchange of the publications and all manuscripts should be addressed to: Managing Editor, Bulletin; Florida State Museum; University of Florida; Gainesville, FL 32611, U.S.A. Copyright © by the Florida State Museum of the University of Florida This public document was promulgated at an annual cost of $3,040.00 or $3.04 per copy. It makes available to libraries, scholars, and allinterested persons the results of researches in the natural sciences, emphasizing the circum-Caribbean region. Publication dates: 8-15-83 Price: 3.10 NON-MARINE MOLLUSKS OF BORNEO II PULMONATA: PUPILLIDAE, CLAUSILIIDAE III PROSOBRANCHIA: HYDROCENIDAE, HELICINIDAE FRED G. THOMPSON AND S. PETER DANCEl ABSTRACT: The Bornean land snails of the families Pupillidae, Clausiliidae, Hydrocenidae, and Helicinidae are reviewed based on collections from38 localities in Sarawak and Sabah and on previous records from the island. The following species are recorded: PUPILLIDAE- Pupisoma orcula (Benson), Costigo putuiusculum (Issel) new combination, Costigo molecul- ina Benthem-Jutting, Nesopupa moreleti (Brown), N. malayana Issel; Boysidia (Dasypupa) salpimf new subgenus and species, B. -

Abstract Volume
ABSTRACT VOLUME August 11-16, 2019 1 2 Table of Contents Pages Acknowledgements……………………………………………………………………………………………...1 Abstracts Symposia and Contributed talks……………………….……………………………………………3-225 Poster Presentations…………………………………………………………………………………226-291 3 Venom Evolution of West African Cone Snails (Gastropoda: Conidae) Samuel Abalde*1, Manuel J. Tenorio2, Carlos M. L. Afonso3, and Rafael Zardoya1 1Museo Nacional de Ciencias Naturales (MNCN-CSIC), Departamento de Biodiversidad y Biologia Evolutiva 2Universidad de Cadiz, Departamento CMIM y Química Inorgánica – Instituto de Biomoléculas (INBIO) 3Universidade do Algarve, Centre of Marine Sciences (CCMAR) Cone snails form one of the most diverse families of marine animals, including more than 900 species classified into almost ninety different (sub)genera. Conids are well known for being active predators on worms, fishes, and even other snails. Cones are venomous gastropods, meaning that they use a sophisticated cocktail of hundreds of toxins, named conotoxins, to subdue their prey. Although this venom has been studied for decades, most of the effort has been focused on Indo-Pacific species. Thus far, Atlantic species have received little attention despite recent radiations have led to a hotspot of diversity in West Africa, with high levels of endemic species. In fact, the Atlantic Chelyconus ermineus is thought to represent an adaptation to piscivory independent from the Indo-Pacific species and is, therefore, key to understanding the basis of this diet specialization. We studied the transcriptomes of the venom gland of three individuals of C. ermineus. The venom repertoire of this species included more than 300 conotoxin precursors, which could be ascribed to 33 known and 22 new (unassigned) protein superfamilies, respectively. Most abundant superfamilies were T, W, O1, M, O2, and Z, accounting for 57% of all detected diversity. -

Citizen Science Using People Power to Map and Monitor Ants
NOVEMBER/DECEMBER 2017 Landscape Insects and Mites | New Guinea Flatworm Learn the Latest PESTPRO Insecticides From Pest Management Education, Inc. to Landscape and Pest Managers Plants, Flooding and Salt Damage Citizen Science Using People Power To Map and Monitor Ants November/December 2017 | PestPro 1 TAURUS® SC Fipronil 9.1% Guaranteed Results 10 YEAR Promise of Protection ControlSolutionsInc.com Taurus is a registered trademark of Control Solutions, Inc. Control Solutions, Inc. Adama.com This product may not be registered in all states, Innovation you can apply. Find Us On please check the CSI website for registration information. 2 PestPro | November/December 2017 VOL. 13, NO. 6 November–December 2017 PESTPRO CONTENTS magazine is a publication of FEATURES Pest Management Education, Inc., and is the official magazine of the Times and Insecticides Florida Pest Management Association Are Changing 8 Citizen Science: Using People Power 11 To Map and Monitor Ants Board of Directors Tim Brock, Brock Lawn & Pest Control New Guinea John Cooksey, McCall Service Flatworm Dr. Phil Koehler, University of Florida 16 Marie Knox, Control Solutions, Inc. Jane Medley, Pest Management Education Student Profile: John Paige III, Bayer Dallin Ashby Dr. Roberto Pereira, University of Florida 18 Sandee Weston, Pest Management Education Tony Weston, Pest Management Education Insects and Mites In the Landscape in 2017 Managing Director 21 Philip Koehler (352) 392-2484 [email protected] Plants, Flooding And Salt Damage Managing Editor 31 Roberto Pereira (352) 392-2485 [email protected] DEPARTMENTS Production Editor Jane Medley (352) 871-1809 6 FPMA President’s Message [email protected] 7 Editorial: Pest Pro Community Pulls Together Advertising Manager Sandra Krempasky (904) 679-5615 13 Past President’s Corner: Pamela Jordan Wolf [email protected] 15 Pest Detective: Seed Beetles PESTPRO (ISSN 1553-4693) is published Jan.–Feb., 23 Market Hardware: Start Turning Negative Reviews March–April, May–June, July–Aug., Sept.–Oct., and Nov.–Dec. -

On the Phylogenetic Relationships of the Genus Mexistrophia and of the Family Cerionidae (Gastropoda: Eupulmonata)
THE NAUTILUS 129(4):156–162, 2015 Page 156 On the phylogenetic relationships of the genus Mexistrophia and of the family Cerionidae (Gastropoda: Eupulmonata) M.G. Harasewych Estuardo Lopez-Vera Fred G. Thompson Amanda M. Windsor Instituto de Ciencias del Mar y Limnologia Florida Museum of Natural History Dept. of Invertebrate Zoology, MRC-163 Universidad Nacional Autonoma de Mexico University of Florida National Museum of Natural History Circuito Exterior S/N Gainesville, FL 32611 USA Smithsonian Institution Ciudad Universitaria PO Box 37012 Delegacion Coyoacan Washington, DC 20013-7012 USA CP: 04510 Mexico D.F. MEXICO [email protected] ABSTRACT morphology, anatomy, and radula of Mexistrophia reticulata, the type species of Mexistrophia,withthoseof Phylogenetic analyses of partial DNA sequences of the mito- several species of Cerion,includingCerion uva (Linnaeus, chondrial COI and 16S rDNA genes derived from Mexistrophia 1758), the type species of the type genus of Cerionidae. reticulata Thompson, 2011, the type species of the genus He concluded that anatomical features of Mexistrophia Mexistrophia, indicate that this genus is sister taxon to all remaining living Cerionidae, and that the family Cerionidae is reticulata are typical of Cerionidae and that radular mor- most closely related to Urocoptidae. Relationships among repre- phology differs only slightly. However, Mexistrophia may sentative cerionid taxa are consistent with the zoogeographic be distinguished from species of Cerion in lacking lamellae hypothesis that Mexistrophia has been isolated from the remain- and denticles along the columella at all stages of growth. ing living Cerionidae since the Cretaceous, and suggest that the Harasewych (2012) reviewed the diversity of living and near-shore, halophilic habitat that has commonly been associated fossil Cerionidae from geographic and temporal perspec- with this family is likely a Cenozoic adaptation that coincided tives and combined these data with paleogeographic recon- with the transition from continental to island habitats. -
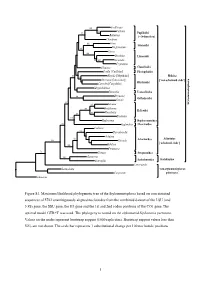
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

A New Record and Developmental Stages of Paropeas Achatinacium (L.P Feiffer, 1846), (Gastropoda; Subulinidea) in Iraq
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2015): 78.96 | Impact Factor (2015): 6.391 A New Record and Developmental Stages of Paropeas achatinacium (L.P Feiffer, 1846), (Gastropoda; Subulinidea) in Iraq Nibrass Lafta Al-doori1, Maysaloon Lafta Al-doori2, Harith Qasim Al- Juburi3, Amal Jabbar4 Dept. of Biology College of Education for Pure Sciences (Ibn-Al-Haitham ) / University of Baghdad / Republic of Iraq Abstract: This study found a new record species Paropeas achatinaceum (L.P Feiffer,1846) In Iraq , which is a terrestrial Molluscan., we studied it for three months (Jun, July, August) in some orchards in Baghdad , A special area has been selected in its environment to identify the stages of growth from egg to adulthood , These stages were documented by taking photographs of each of them , we found that it need (2 – 3) months to been adult and it may live for a year , and lay (20 – 40) eggs in gelatinous sac . Keywords: 1. Introduction we found this species which have characters include shell small elongate (11 mm), clear yellow (Fig.1: a,b), but Bash The gastropods (snails and slugs) are distributed among [11] record that the shell have pale yellow. P. achatinaceum freshwater, marine water and terrestrial environment , these has eight right convex whorls with semi deep structures; the animals is growing on decadent plants and feeding on many top of the shell was not sharp. The aperture has oval shape, species, but some of them used to be carnivorours [1]. There oblique and small. The aperture has rounded shape in the is a gap information about the basic biology of the land base and pointed shape in the top (4.4x2.3) mm, which has a snail. -

Occasional Papers
NUMBER 121, 11 pages 17 August 2017 BISHOP MUSEUM OCCASIONAL PAPERS FIRST RECORDS OF THE INVASIVE PREDATORY LAND SNAIL GULELLA (HUTTONELLA ) BICOLOR (H UTTON , 1834) (G ASTROPODA : STREPTAXIDAE ) FROM THE SOCIETY ISLANDS , FRENCH POLYNESIA CARL C. C HRISTENSEN & J ENNIFER G. K AHN BISHOP MUSEUM PRESS HONOLULU Cover image: Photo of site ScMo-350 in Haumi Bay, Mo'orea, Society Islands. Photo: J. Kahn. Bishop Museum Press has been publishing scholarly books on the natu - ESEARCH ral and cultural history of Hawai‘i and the Pacific since 1892. The R Bishop Museum Occasional Papers (eISSN 2376-3191) is a series of short papers describing original research in the natural and cultural sci - PUBLICATIONS OF ences. BISHOP MUSEUM The Bishop Museum Press also publishes the Bishop Museum Bulletin series. It was begun in 1922 as a series of monographs presenting the results of research throughout the Pacific in many scientific fields. In 1987, the Bulletin series was separated into the Museum’s five current monographic series, issued irregularly and, since 2017, electronically: Bishop Museum Bulletins in Anthropology (eISSN 2376-3132) Bishop Museum Bulletins in Botany (eISSN 2376-3078) Bishop Museum Bulletins in Entomology (eISSN 2376-3124) Bishop Museum Bulletins in Zoology (eISSN 2376-3213) Bishop Museum Bulletins in Cultural and Environmental Studies (eISSN 2376-3159) To subscribe to any of the above series, or to purchase individual publi - cations, please write to: Bishop Museum Press, 1525 Bernice Street, Honolulu, Hawai‘i 96817-2704, USA. Phone: (808) 848-4135. Email: [email protected]. BERNICE PAUAHI BISHOP MUSEUM ISSN 0893-1348 (print) The State Museum of Natural and Cultural History ISSN 2376-3191 (online) 1525 Bernice Street Copyright © by Bishop Museum Honolulu, Hawai‘i 96817-2704, USA Published online: 17 August 2017 ISSN (online): 2376-3191 First Records of the Invasive Predatory Land Snail Gulella (Huttonella) bicolor (Hutton, 1834) (Gastropoda: Streptaxidae) from the Society Islands, French Polynesia . -

ZM82 615-650 Robinson.Indd
The land Mollusca of Dominica (Lesser Antilles), with notes on some enigmatic or rare species D.G. Robinson, A. Hovestadt, A. Fields & A.S.H. Breure Robinson, D.G., A. Hovestadt, A. Fields & A.S.H. Breure. The land Mollusca of Dominica, Lesser Antilles, with notes on some enigmatic or rare species. Zool. Med. Leiden 83 (13), 9.vii.2009: 615-650, fi gs 1-14, tables 1-2.― ISSN 0024-0672. D.G. Robinson, The Academy of Natural Sciences, 1900 Benjamin Franklin Parkway, Philadelphia, PA 19103, U.S.A. ([email protected]). A. Hovestadt, Dr. Abraham Kuyperlaan 22, NL-3818 JC Amersfoort, The Netherlands (ad.hovestadt@ xs4all.nl). A. Fields, Department of Biological and Chemical Sciences, University of the West Indies, Cave Hill Cam- pus, Barbados (angela.fi [email protected]). A.S.H. Breure, National Museum of Natural History, P.O. Box 9517, NL-2300 RA Leiden, The Netherlands ([email protected]). Key words: Mollusca, Gastropoda, taxonomy, distribution, Dominica. An overview of the land-snail fauna of the Lesser Antillean island of Dominica is given, based on data from literature and four recent surveys. There are 42 taxa listed, of which the following species are recorded for the fi rst time from the island: Allopeas gracile (Hutt on, 1834), A. micra (d’Orbigny, 1835), Beckianum beckianum (L. Pfeiff er, 1846), Bulimulus diaphanus fraterculus (Potiez & Michaud, 1835), De- roceras laeve (Müller, 1774), Sarasinula marginata (Semper, 1885), Streptostele musaecola (Morelet, 1860) and Veronicella sloanii (Cuvier, 1817). The enigmatic Bulimulus stenogyroides Guppy, 1868 is now placed in the genus Naesiotus Albers, 1850.