View Different Locations Mix Homogeneously
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Aalborg Universitet Restructuring State and Society Ethnic
Aalborg Universitet Restructuring State and Society Ethnic Federalism in Ethiopia Balcha, Berhanu Publication date: 2007 Document Version Publisher's PDF, also known as Version of record Link to publication from Aalborg University Citation for published version (APA): Balcha, B. (2007). Restructuring State and Society: Ethnic Federalism in Ethiopia. SPIRIT. Spirit PhD Series No. 8 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. ? Users may download and print one copy of any publication from the public portal for the purpose of private study or research. ? You may not further distribute the material or use it for any profit-making activity or commercial gain ? You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us at [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from vbn.aau.dk on: November 29, 2020 SPIRIT Doctoral Programme Aalborg University Kroghstraede 3-3.237 DK-9220 Aalborg East Phone: +45 9940 9810 Mail: [email protected] Restructuring State and Society: Ethnic Federalism in Ethiopia Berhanu Gutema Balcha SPIRIT PhD Series Thesis no. 8 ISSN: 1903-7783 © 2007 Berhanu Gutema Balcha Restructuring State and Society: Ethnic Federalism in Ethiopia SPIRIT – Doctoral Programme Aalborg University Denmark SPIRIT PhD Series Thesis no. -

Patriotic Resistance Against Italian Invasion in Sadan Sooddoo Oromo (1936-41)
PJAEE, 17 (9) (2020) Patriotic resistance against Italian invasion in Sadan Sooddoo Oromo (1936-41) Gemechu Kenea 1, Surafel Adissu 2 1College of Social science and Humanities, Department of History and Heritage Management, Bule Hora University 2College of Social sciences and Humanities, Department of Social Anthropology, Jimma University Email:1 [email protected], [email protected] Gemechu Kenea , Surafel Adissu 2: Patriotic resistance against Italian invasion in Sadan Sooddoo Oromo (1936-41)-- Palarch’s Journal Of Archaeology Of Egypt/Egyptology 17(9). ISSN 1567-214x Keywords: Oromo, Sadan Sooddoo, resistance, Italy, patriots. ABSTRACT The aim of this paper is to highlight the resistance made by Sadan Sooddoo Oromo patriots against Italians during 1936-41. Qualitative research methodology with Purposive sampling technique was employed in this study and the relevant respondents from the target group were selected carefully by the researcher to get reliable and rich data. Available primary and secondary sources, the paper seriously took those in to account. In this paper the Sadan Sooddoo resistance, the notable partiots and the battle fought with Italians during the period was discussed and analyzed. This study casts some light on how the patriots made struggle against colonizer. This paper argued that, even though Haile Sillasie I was fled to outside after the battle of Maichew the patriots including Sadan Sooddo Oromo patriots pay great scarification and made struggle against Italian colony until the liberation of 1941. 1. Introduction The Oromo of Sadan Sooddoo are named as such because they are three in number. They are Odituu (the elder), Tummee and Liiban. They are generally settled in the areas to the South and South West of the overall settlement of the Tulamaa with an extension to the area south of the Awash river as far as Arsi and the Borana of the Southern part of Oromia. -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -
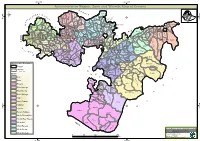
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm. -

Nutritional Causal Analysis East Hararghe Zone, Fedis and Kersa Woredas, Ethiopia, August, 2014
East Hararghe Zone, Fedis and Kersa Woredas, Ethiopia Action Contre La Faim_ Ethiopia mission Nutritional Causal Analysis East Hararghe Zone, Fedis and Kersa Woredas, Ethiopia, August, 2014 Carine Magen, Health Anthropologist, and ACF team, Ethiopia mission 11/1/2014 ACF East Hararghe Nutrition Causal Analysis Report Page 1 LIST OF ACRONYMS ARI Acute Respiratory Infection BCG Bacillus Calmette Guerin CBN Community Based Nutrition CGC Charcher, Gololicha zone (Coffee, Khat, Maize) CVG Khat, Vegetable CI Confidence Interval CMAM Community-based Management of Acute Malnutrition CDR Crude Death Rate CHD Community Health Day CLTS Community Led Total Sanitation CSB Corn Soya Blended food DE Design Effect DPPO Disaster Preparedness and Prevention Office DRMFSS Disaster Risk Management and Food Security Sector ENA Emergency Nutrition Assessment ENCU Emergency Nutrition Coordination Unit EPI Extended Programme of Immunization ETB Ethiopian Birr FGD Focus Group Discussion GAM Global Acute Malnutrition GBG Gursum and Babile zone (sorghum, maize, haricot bean) HH Households HRF Humanitarian Response Fund IGA Income generating activities IMC International Medical Corps IYCF Infant and Young Child Feeding MAM Moderate Acute Malnutrition MNC Mother with Malnourished Child MUAC Mid-Upper Arm Circumference NCA Nutrition Causal Analysis NNP National Nutrition program NCHS National Centre for Health Statistics ODPPC Oromiya Disaster Prevention and Preparedness Commission OTP Out-Patient Therapeutic Program PPS Probability Proportional to Population Size -

In Case of Dawo District of South West Shewa Zone
Kebede Lemu Bekelcha and Aregash Eticha Sefera, GJR 2019,1:2 Research Article GJR 2019,1:2 Global Journal of Religions (DOI:10.28933/GJR) The Role of Customary Conflict Resolution Mechanisms Among the Oromo: In Case of Dawo District of South West Shewa Zone Kebede Lemu Bekelcha1, Aregash Eticha Sefera2 1 Lecturer at Bule Hora University, Faculty of Social Sciences and Humanities, Social Anthropology Department, Bule Hora, Ethiopia. 2 Lecturer at Bule Hora University, Faculty of Social Sciences and Humanities, Department of Social Anthropology, Bule Hora, Ethiopia. ABSTRACT This study deals with the role of customary conflict resolution *Correspondence to Author: mechanisms in Oromia region with particular emphasis on Dawo Kebede Lemu Bekelcha district of south west shewa zone. Hence, the (i) purpose of this Lecturer at Bule Hora University, study was to examine and explore the significance of customary Faculty of Social Sciences and Hu- conflict resolution mechanisms in anthropological perspectives manities, Social Anthropology De- in the study area. The (ii) intent of the study was to identify the partment, Bule Hora, Ethiopia types of customary conflict resolution mechanisms and cause of conflict in Dawo district. The (iii) purpose of the study was to describe the structure and procedure as well as advantage and How to cite this article: disadvantages of customary conflict resolution mechanisms. Kebede Lemu Bekelcha, Aregash Finally, the purpose of this study was to provide information to Eticha Sefera.The Role of Custom- understanding about the study area. To achieve this objective, ary Conflict Resolution Mechanisms both primary and secondary data was used. This study applied Among the Oromo: In Case of qualitative data. -

Ethiopia Administrative Map As of 2013
(as of 27 March 2013) ETHIOPIA:Administrative Map R E Legend E R I T R E A North D Western \( Erob \ Tahtay Laelay National Capital Mereb Ahferom Gulomekeda Adiyabo Adiyabo Leke Central Ganta S Dalul P Afeshum Saesie Tahtay Laelay Adwa E P Tahtay Tsaedaemba Regional Capital Kafta Maychew Maychew Koraro Humera Asgede Werei Eastern A Leke Hawzen Tsimbila Medebay Koneba Zana Kelete Berahle Western Atsbi International Boundary Welkait Awelallo Naeder Tigray Wenberta Tselemti Adet Kola Degua Tsegede Temben Mekele Temben P Zone 2 Undetermined Boundary Addi Tselemt Tanqua Afdera Abergele Enderta Arekay Ab Ala Tsegede Beyeda Mirab Armacho Debark Hintalo Abergele Saharti Erebti Regional Boundary Wejirat Tach Samre Megale Bidu Armacho Dabat Janamora Alaje Lay Sahla Zonal Boundary Armacho Wegera Southern Ziquala Metema Sekota Endamehoni Raya S U D A N North Wag Azebo Chilga Yalo Amhara East Ofla Teru Woreda Boundary Gonder West Belesa Himra Kurri Gonder Dehana Dembia Belesa Zuria Gaz Alamata Zone 4 Quara Gibla Elidar Takusa I Libo Ebenat Gulina Lake Kemkem Bugna Kobo Awra Afar T Lake Tana Lasta Gidan (Ayna) Zone 1 0 50 100 200 km Alfa Ewa U Fogera North Farta Lay Semera ¹ Meket Guba Lafto Semen Gayint Wollo P O Dubti Jawi Achefer Bahir Dar East Tach Wadla Habru Chifra B G U L F O F A D E N Delanta Aysaita Creation date:27 Mar.2013 P Dera Esite Gayint I Debub Bahirdar Ambasel Dawunt Worebabu Map Doc Name:21_ADM_000_ETH_032713_A0 Achefer Zuria West Thehulederie J Dangura Simada Tenta Sources:CSA (2007 population census purpose) and Field Pawe Mecha -

Assessments of Community‟S Perception Toward the Practices of Female Genital Mutilation in Oromia Regional State: the Case of Wonchi Woreda, Central Ethiopia
ASSESSMENTS OF COMMUNITY‟S PERCEPTION TOWARD THE PRACTICES OF FEMALE GENITAL MUTILATION IN OROMIA REGIONAL STATE: THE CASE OF WONCHI WOREDA, CENTRAL ETHIOPIA MA THESIS BY KEBEDE WOLDE MULETA AUGUST, 2018 ARBA MINCH, ETHIOPIA i ASSESSMENTS OF COMMUNITY‟S PERCEPTION TOWARD THE PRACTICES OF FEMALE GENITAL MUTILATION IN OROMIA REGIONAL STATE: THE CASE OF WONCHI WOREDA, CENTRAL ETHIOPIA BY: KEBEDE WOLDE MULETA A THESIS SUBMITTED TO DEPARTMENT OF GEOGRAPHY AND ENVIRONMENTAL STUDIES, COLLEGE OF SOCIAL SCIENCE AND HUMANITYS, SCHOOL OF GRADUATE STUDIES, ARBA MINCH UNIVERSITY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS IN GEOGRAPHY AUGUST, 2018 ARBA MINCH, ETHIOPIA Declaration I hereby declare that this MA thesis is my original work and has not been presented for a degree in any other university, and all sources of material used for this thesis have been duly acknowledged. Name: Kebede Wolde Signature: ______________________ Date: __________________________ SCHOOL OF GRADUATE STUDIES ARBA MINCH UNIVERSITY ADVISORS‟ THESIS SUBMISSION APPROVAL SHEET This is to certify that the thesis entitled “Assessments of Community’s Perception toward the Practices of Female Genital Mutilation” submitted in partial fulfillment of the requirements for the degree of Master‟s in the Graduate Program of the Department of Geography and environmental Studies and has been carried out by Kebede Wolde Id. No SMA /018/2006 under my supervision. Therefore I recommend that the student has fulfilled the requirements and hence hereby can submit -

Partial List of Oromos Mainly Students That Have Been Killed By
Sochii Dargaggoota Biyyoolessaa, National Youth Movement for Bilisummaa fi Dimokraasiif(SDBBD) Freedom and Democracy (NYMFD) Partial list of Oromos mainly students that have been killed by Ethiopian regime police, security agents, Special and armed force during peaceful demonstration of last three weeks (updated stand. 26 Dece. 2015) No. Name Place of Birth/zone School/Universit Place Killed Date of Death Killed by y attending 1 Gazahany Oliiqaa Horro Guduru Haromaya Haromaya Dece.1/2015 Federal police th 2 Guutu Abarra Dheeressa W. Wollega, Gulliso 12 grade Gulliso Dece.2/2015 Federal police 3 Karrasa Chala W. Wollega, Gulliso 12th grade Gulliso Dece. 2/2015 Federal police 4 Dabala Tafa Robi N. Shewa, Fiche ---- Chancho Dece. 2/2015 Federal police 5 Dajane Sarbeessa S. W. Shoa, Tole Secondary School Tole Dece. 3/2015 Federal police 6 Miftah Junedin Bushura Hararge, Baddanno Secondary School Farad Dece. 5/2015 Federal police 7 Murata Abdi Ibrahim Haromaya, Gutto 11th grade Haromaya Dece. 5/2015 Federal police 8 Bekkele Sabbooqaa W. Shewa. Ada Barga Secondary School Muger Dece. 8/2015 Police 9 Bekele Seifu W. Shewa. Ada Barga Muger Dece. 8/2015 Police 10 Eebbisa Bucho E.Wallagaa Employee Fincha’a Dece. 8/2015 Police 11 Bayisa Badhasa West Seawa, Cheliya 1st year at Bako Bako Dece.9/2015 Agazi Agriculture College 12 Gudeta Bayisa Gobena W.Shewa,Tullu bajjo 9th grade Babichi Dece.10/2015 Agazi 13 Chala Tashoma West Shewa Babichi Dece10/2015 Agazi 14 Bayissa Tadesse W. Shawa, Abuna Dece.10/2015 Agazi Gindabarat 15 Bu’e Dhaba W.Shewa Abuna -

Downloads (Not All App Sharing and Downloads Could Be Tracked)
m.<i Oll'lf'ltC- 1\.-\-V-A-i MINISTRY OF HEALTH·ETHIOPIA r11,•H·· rn.'i h'/1c. •OM)·l•i! USAID @ §.filQ Gt HEALTHIER CrrtZENS FOR PROSPEROUS NATION! FROM THE AMERICAN PEOPLE ACTIVITY INFORMATION Activity Title Communication for Health [Contract/Agreement] Number AID-663-A-15-000011 Name of Prime Implementing Partner Johns Hopkins Center for Communication Programs Name(s) of Subcontractor(s)/Sub awardee(s) John Snow, Inc. Activity Start Date July 20, 2015 Activity End Date December 31, 2020 Reporting Period July 20, 2015– December 31, 2020 Budget $22,193,954.00 Communication for Health 2 CONTENTS ACTIVITY INFORMATION . 2 Contents . 3 Acronyms and Abbreviations . 4 EXECUTIVE SUMMARY . 5 PROJECT OVERVIEW . 6 Theoretical Approach . 6 Project Goal, Objectives and Strategies . 7 Project Timeline and Focus Areas . 9 Partners . 10 IR1: STRENGTHENED PUBLIC HEALTH SYSTEMS AND COORDINATION FOR SBC . .. .11 IR2: SBCC DESIGN AND IMPLEMENTATION STRENGTHENED . ..17 IR 3: IMPROVED DATA USE FOR DECISION-MAKING . .. .. .. 28 CROSS-CUTTING ISSUES . 31 Gender Equality and Women Empowerment . ..31 Stakeholder Collaboration . .33 Collaboration and Knowledge Sharing with Other USAID Activities . 33 Collaboration and Coordination with Other Key Stakeholders . 33 Project Monitoring and Evaluation . 36 SUMMARY OF RESULTS . 36 CHALLENGES AND LESSONS LEARNED . 37 Appendixes . 38 End of Project Report (2015-2020) 3 Acronyms and Abbreviations AWD Acute Watery Diarrhea CCP Johns Hopkins Center for Communication Programs COP Community of Practice COR/AOR Country Officer’s