Before the Scientific and Medical Accountability
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bedford Stem Cell Research Foundation
bedford stem cell research foundation ||| The Potential for Miracles ||| Recent advances in stem cell research have raised the hope Founded in 1996, the Bedford Stem Cell of curing diseases once believed to be incurable: heart failure, spinal cord injury, diabetes, Alzheimer’s, Parkinson’s, Research Foundation is a biomedical AIDS. These diseases are the result of the death of specifi c types of cells, such as nerves and the cells in the pancreas institute that exists to conduct stem that produce insulin. For reasons that are not understood, cell and related research for diseases new cells do not automatically replace defective cells in some tissues such as spinal cord, brain and pancreas. and conditions that currently have no Other tissues, such as skin and blood, routinely replace effective methods of treatment or cure. dying cells with new cells recruited from reserve supplies that maintain the potential to become active and multiply when needed. [fi g 1] Such cells are called stem cells. Skin stem cells are examples of adult stem cells, so-called ||| The Foundation ||| because they can become only one type of tissue, e.g. skin. In contrast, cells from early embryos are pluripotent, that Bedford Stem Cell Research Foundation is at the forefront is, they have the potential to become all types of tissues. of stem cell and related research. Founded in 1996, the Experiments with laboratory mice have demonstrated that Foundation has an established community of scientists pluripotent stem cells can replace dead cells in all organs investigating stem cell therapies. Committed to conduct- including the heart, which does not have its own supply of ing ethical research, the Foundation relies on its Ethics stem cells. -

Before the Scientific and Medical Accountability
BEFORE THE SCIENTIFIC AND MEDICAL ACCOUNTABILITY STANDARDS WORKING GROUP OF THE INDEPENDENT CITIZENS' OVERSIGHT COMMITTEE TO THE CALIFORNIA INSTITUTE FOR REGENERATIVE MEDICINE ORGANIZED PURSUANT TO THE CALIFORNIA STEM CELL RESEARCH AND CURES ACT REGULAR MEETING LOCATION: AS INDICATED ON THE AGENDA DATE: JULY 24, 2013 10 A.M. REPORTER: BETH C. DRAIN, CSR CSR. NO. 7152 BRS FILE NO.: 94597 BARRISTERS' REPORTING SERVICE I N D E X ITEM DESCRIPTION PAGE NO. CALL TO ORDER 3 ROLL CALL 3 CONSIDERATION OF IMTERIM REGULATION REGARDING USE OF STEM CELL LINES PUBLIC COMMENT 2 160 S. OLD SPRINGS ROAD, SUITE 270, ANAHEIM, CALIFORNIA 92808 1-800-622-6092 1-714-444-4100 EMAIL: [email protected] BARRISTERS' REPORTING SERVICE 1 SAN FRANCISCO, CALIFORNIA; JULY 24, 2013 2 12 P.M. 3 4 DR. LOMAX: WHY DON'T WE START WITH ROLL. 5 IT'S ONLY ONE PERSON WHO'S RESPONDED THAT I KNOW IS 6 NOT HERE ON-SITE, BUT HOPEFULLY TED PETERS WILL BE 7 ARRIVING SHORTLY. 8 SHERRY LANSING. 9 MS. LANSING: HERE. 10 DR. LOMAX: BERNARD LO. 11 CHAIRMAN LO: HERE. 12 DR. LOMAX: JEFF BODKIN. 13 DR. BODKIN: HERE. 14 DR. LOMAX: MARCY FEIT. TIM KAMP. 15 DR. KAMP: YES. 16 DR. LOMAX: ANN KIESSLING. KEN OLDEN. 17 FRANCISCO PRIETO. 18 DR. PRIETO: HERE. 19 DR. LOMAX: TED PETERS. 20 DR. PETERS: TED PETERS IS HERE, YES, ON 21 THE PHONE. 22 DR. LOMAX: OH, TERRIFIC. DOROTHY 23 ROBERTS. 24 DR. ROBERTS: YES. I'M HERE ON THE PHONE. 25 DR. LOMAX: TERRIFIC. 3 160 S. OLD SPRINGS ROAD, SUITE 270, ANAHEIM, CALIFORNIA 92808 1-800-622-6092 1-714-444-4100 EMAIL: [email protected] BARRISTERS' REPORTING SERVICE 1 JEFF SHEEHY. -

BEDFORD STEM CELL RESEARCH FOUNDATION Massachusetts 501(C)(3) Not for Profit Organization P: 617-281-7902 E: [email protected] W
BEDFORD STEM CELL RESEARCH FOUNDATION Massachusetts 501(c)(3) not for profit organization P: 617-281-7902 E: [email protected] W: www.bedfordresearch.org The Testis: a source of stem cells for all men? Could the newly discovered “testis stem cells” be as versatile as embryonic stem cells? Bedford is launching a major research initiative to find out. A major barrier to progress in stem cell avoid the rejection that occurs if therapies is that, similar to organ transferred cells are not a perfect tissue transplantation, the body rejects cells match to the patient’s own cells. that are not a good match for the patient. Surprisingly, stem cells from testis may Dr. Martin Dym’s research team at $280k offer a solution. In 2008, a German team Georgetown University estimates reported that they successfully derived approximately 1 in 100,000 testis stem $250K pluripotent stem cells from adult mouse cells may be pluripotent. For several $200k testis. Because of their unique ability to decades, it has been known that sperm become any type of cell in the body, are abundantly produced (billions per $150k pluripotent stem cells hold the promise of week) in the adult male testis for life, cures to many conditions, from diabetes and that the sperm arise from “sperm $136k to spinal cord diseases. Then in 2009, stem cells” termed spermatogonia. two U.S. research teams discovered However, the new reports indicate that $100k pluripotent stem cells in human testis. in addition to the spermatogonia, there $50k are pluripotent stem cells in the testis. To If pluripotent stem follow up these exciting reports, Bedford $0 cells can be derived Research scientists have launched a new from every human research initiative to answer several testis, it could launch a important questions: (1) Can stem cells Mouse Testis new era in stem cell be derived from the testis of all men? Stem Cells research. -

Words from the Chairman
Department of Surgery Annual Research Report 2007 Words from the Chairman Surgical Research is part of any contemporary, first class, Department of Surgery and the Beth Israel Deaconess Medical Center is no exception. As the Department grew, so did its research effort and level of funding. Until recently, continual growth in research funding for the Department of Surgery was gratifying indeed. The Department’s research funding from all sources, both NIH and non-NIH, increased from about $10 to more than $21 million annually, during the period of 2002-2005. This growth was impressive, taking the Department from approximately 22nd in the country in total NIH funding, to about 5th or 6th. It is difficult to obtain our exact position amongst NIH-funded Departments of Surgery because this number is calculated by the NIH as a total of all Departments of Surgery at Harvard Medical School, including totals for all Harvard affiliated hospitals. Since 2005, our NIH and other funding for research have been declining. The NIH seems to view its funding for research as discretionary, which is particularly unfortunate given the number of people and planning that must go in to building one’s research holdings, and also the nature of individuals that need to be supported. When there is little money, as there currently is not going to various military needs and other non-discretionary activity, NIH funding declines. Just as others have experienced a downturn in NIH funding, so have we. Our NIH and other funding have diminished, including a reduction in the total dollar amount for each awarded grant and the total number of NIH grants that are funded, including competing renewals of long-standing projects. -

M.Sc Ist Yr MICROBIOLOGY a Bottle of Wine Contains More Philosophy Than All the Books in the World
M.Sc Ist yr MICROBIOLOGY A bottle of wine contains more philosophy than all the books in the world. -Louis Pasteur JANUARY 2022 M T W T F S S Ferdinand Cohn (Founder of 31 1 2 Bacteriology and Microbiology) Global Family Day 3 4 5 6 7 8 9 Richard Rebecca Har Michael Craighill Gobind Krause Lancefield Khorana Rebecca Craighill Lancefield (well known for serological classification of β- hemolytic streptococcal bacteria) 10 11 12 13 14 15 16 National Indian Youth Day Army Day 17 18 19 20 21 22 23 Bacillus cereus Bacillus cereus is a facultatively anaerobic, toxin producing, gram- positive bacteria that canm be found in siol vegetation anf even food. This may cause two types of intestinal illness, one diarrheal, and 24 25 26 27 28 29 30 one causing nausea and vomiting.It Ferdinand National Heinrich World can quickly multiply at room Kohn Tourism Anton de Leprosy temperature. B.cereus has also been National Girl Day Bary Day Child Day Republic implicated in infections of the eye, respiratory tracts , and in wounds. International Day B.cereus and other members of Day of Education bacillus are not easily killed by alcohol,they have been known to World Leprosy Day – 30th JAUNARY: World Leprosy colonize distilled liquors and Day is observed internationally every year on the last Sunday of alcohol soaked swabs and pads in January to increase the public awareness of leprosy or Hansen's numbers sufficient to cause Disease. This date was chosen by French humanitarian Raoul infection. Follereau as a tribute to the life of Mahatma Gandhi who had compassion for people afflicted with leprosy. -

Ann Kiessling Oral History Interview, “Reflections on Oregon Roots”, June 13, 2014 Page 2 of 11
Ann Kiessling Oral History Interview, June 13, 2014 Title “Reflections on Oregon Roots” Date June 13, 2014 Location Valley Library, Oregon State University. Summary In the interview, Kiessling speaks of her connections with the state of Oregon and OSU. In so doing, she reflects on the academic path that led to her arrival at OSU as a doctoral candidate in 1967, a path that included her earning a degree in nursing as a tool for funding further schooling. She discusses her association with her major professor at OSU, Dr. George Beaudreau, and the work that she conducted in his laboratory, investigating the relationship between viruses and cancer. She shares her memories of her academic progression at Oregon State as well as campus and community life in the late 1960s and early 1970s. She likewise notes the impact that OSU made on the next stages of her career. The remainder of the interview is chiefly devoted to Kiessling's recollections of her broader ties to the state, including her upbringing in Klamath Falls and the eight years that she spent on faculty at Oregon Health Sciences University. This interview was conducted in connection with Kiessling's June 2014 visit to Corvallis, during which time she delivered the Commencement Address at OSU's graduation exercises. Interviewee Ann Kiessling Interviewer Chris Petersen Website http://scarc.library.oregonstate.edu/oh150/kiessling/ PDF Created November 16, 2017 Ann Kiessling Oral History Interview, “Reflections on Oregon Roots”, June 13, 2014 Page 2 of 11 Transcript Chris Petersen: Okay, if you could please introduce yourself with your name and today's date, and our location? Anne Kiessling: I'm Ann Kiessling, and this is June 13th, 2014, and we are somewhere in the basement of the OSU library. -

Research Biotechnology Clarify the Ethics of Stem Cell a Natural Stem
Downloaded from jme.bmjjournals.com on 8 May 2006 A natural stem cell therapy? How novel findings and biotechnology clarify the ethics of stem cell research P Patel J. Med. Ethics 2006;32;235-239 doi:10.1136/jme.2005.012096 Updated information and services can be found at: http://jme.bmjjournals.com/cgi/content/full/32/4/235 These include: References This article cites 18 articles, 7 of which can be accessed free at: http://jme.bmjjournals.com/cgi/content/full/32/4/235#BIBL Rapid responses One rapid response has been posted to this article, which you can access for free at: http://jme.bmjjournals.com/cgi/content/full/32/4/235#responses You can respond to this article at: http://jme.bmjjournals.com/cgi/eletter-submit/32/4/235 Email alerting Receive free email alerts when new articles cite this article - sign up in the box at the service top right corner of the article Topic collections Articles on similar topics can be found in the following collections Other Ethics (1359 articles) Research and publication ethics (478 articles) Notes To order reprints of this article go to: http://www.bmjjournals.com/cgi/reprintform To subscribe to Journal of Medical Ethics go to: http://www.bmjjournals.com/subscriptions/ Downloaded from jme.bmjjournals.com on 8 May 2006 235 RESEARCH ETHICS A natural stem cell therapy? How novel findings and biotechnology clarify the ethics of stem cell research P Patel ............................................................................................................................... J Med Ethics 2006;32:235–239. doi: 10.1136/jme.2005.012096 The natural replacement of damaged cells by stem cells occurs actively and often in adult tissues, especially rapidly dividing cells such as blood cells. -
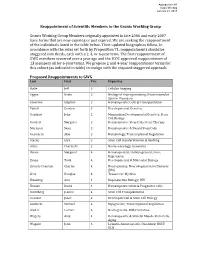
7 Proposed Reappointments to GWG2013
Agenda Item #7 ICOC Meeting January 24, 2013 Reappointment of Scientific Members to the Grants Working Group Grants Working Group Members originally appointed in late 2006 and early 2007 have terms that are now expiring or just expired. We are seeking the reappointment of the individuals listed in the table below. Their updated biographies follow. In accordance with the rules set forth by Proposition 71, reappointments should be staggered into thirds, each with a 2, 4, or 6-year term. The first reappointment of GWG members occurred over a year ago and the ICOC approved reappointment of 13 members all for 6-year terms. We propose 2 and 4-year reappointment terms for this cohort (as indiCated in table) to realign with the required staggered approach. Proposed Reappointments to GWG Last First Yrs. Expertise Bulte Jeff 2 Cellular Imaging Eggan Kevin 2 Biology of Reprogramming; NeuromusCular System Disorders Emerson Stephen 2 HematopoietiC Cells & Transplantation Fishell Gordon 2 Developmental GenetiCs Gearhart John 2 Mammalian Developmental GenetiCs; Stem Cell Biology Goodell Margaret 2 HematopoietiC Stem Cells; Gene Therapy Morrison Sean 2 HematopoietiC & Neural Stem Cells Rosmarin Alan 2 Hematology; TransCriptional Regulation Stacey Glyn 2 Stem Cell Standardization & Banking Stiles Charles D. 2 Neuro-onCology; GenomiCs Baron Margaret 4 Hematopoiesis; Embryogenesis; Gene Expression Evans Todd 4 Developmental & MoleCular Biology ffrenCh-Constant Charles 4 Neurogenesis; Neurodegenerative Diseases (MS) Kerr Douglas 4 Transverse Myelitis Kiessling Ann 4 ReproduCtive Biology; HIV Krause Diane 4 HematopoietiC Stem & Progenitor Cells Kurtzberg Joanne 4 Stem Cell Transplantation Rossant Janet 4 Developmental & Stem Cell Biology RudniCki MiChael 4 Myogenesis; TransCriptional Regulation Studer Lorenz 4 Neurogenesis; Differentiation Wagers Amy 4 HematopoietiC & Skeletal MusCle Stem Cells; Aging Wagner John 4 Lympho-hematopoietiC Disorders; HSCT; UCB Agenda Item #7 ICOC Meeting January 24, 2013 Margaret H. -

Scientific and Medical Standards and Accountability Working Group
CALIFORNIA INSTITUTE FOR REGENERATIVE MEDICINE Scientific and Medical Accountability Standards Working Group Working Group Expertise Affiliation Member Chairman, ICOC N/A Robert Klein Sherry Lansing ICOC Patient Advocate for N/A (Co-Chair) Cancer ICOC Patient Advocate for Type I N/A Francisco Prieto Diabetes ICOC Patient Advocate for N/A Jeff Sheehy HIV/AIDS ICOC Patient Advocate for Jonathan Shestack N/A Mental Health Ethicists Alta Charo Health Law, Bioethics and University of Wisconsin Biotechnology Law, Medical Ethics, Reproductive Rights Bernard Lo Biomedical Ethics related to University of California San oocyte, embryo and stem cell Francisco research Ted Peters Biomedical Ethics of stem cell Pacific Lutheran Theological research and Genetics Seminary, Graduate Theological Union Harriet Rabb Biomedical ethics and Health Rockefeller University (Co-Chair) Law Scientists/Clinicians Jose Cibelli SCNT & Michigan State University Primate Embryonic Stem Cells Kevin Eggan Epigenetics, SCNT Harvard University Ann Kiessling SCNT & Oocyte Derivation, IVF Harvard Institute of Medicine and egg donation Jeffrey Kordower Neurodegenerative Diseases Rush Presbyterian-St. Luke’s Medical Center Kenneth Olden Cellular Biology/Biochemistry, National Institute of hematopoietic SCs Environmental Health Sciences Janet Rowley Oncology, Molecular Genetics, University of Chicago School of and Cell Biology, hematopoietic Medicine SCs Robert Taylor Reproductive biology; IVF and Emory University egg donation James Willerson Stem cell biology (applications to University of Texas Health treat damaged heart tissue); Sciences Center; Texas Heart clinical trials Institute . -

BEDFORD STEM CELL RESEARCH FOUNDATION Massachusetts 501(C)(3) Not for Profit Organization P: 617-281-7902 E: [email protected] W
BEDFORD STEM CELL RESEARCH FOUNDATION Massachusetts 501(c)(3) not for profit organization P: 617-281-7902 E: [email protected] W: www.bedfordresearch.org Bedford Research Stem Cells Glow! Breakthroughs in understanding circadian rhythms in stem cells. Fall 2014: Bedford Research scientists Mouse do, indeed “glow” (Figure 1), and that are following up on their discovery the “glow” actually begins soon after that stem cells have a circadian egg activation, and increases with the rhythm that may need to be sup- + = transition into stem cells (Figure 2). Firefly ported for optimum development in the laboratory. The PER2Luc mouse has circadian genes coupled In the body, the daily pattern of with firefly “Luciferase” genes. When the circadian genes turn “on” they glow, dimly, like a firefly. light and dark controls many sig- nals sent out by the brain, such as Figure 1: PerLuc stem cells those that trigger changes in body Until this fall, Bedford Research scien- glow intermittently temperature, and feelings of hunger tists have been unable to discover the and sleepiness. circadian signal needs of their two new lines of stem cells from the PerLuc Stem cells may especially need cir- mouse because of the lack of a micro- cadian signals to differentiate into spe- scope sensitive enough to detect and cific cell types, such as neurons or photograph the glow of a small num- bone marrow — but what type of sig- Figure 2: New colony of stem cells ber of cells. from a PerLuc embryo nal should they receive in the labora- tory? And what frequency? There is The good news is that such a micro- These exciting new findings provide growing evidence that each type of cell scope has been developed, and this strong support for the importance of needs a different circadian signal. -

Download the Full Program Here (Pdf)
THANK YOU TO TODAY’S SPONSORS THE SEVENTH ANNUAL McKnight Foundation Olympus ctivated gg SYMPOSIUEM Please mail November 6, 2009 FOUNDATION MISSION contributions to: Sponsored by the BEDFORD STEM CELL RESEARCH FOUNDATION Bedford Stem Cell Research Foundation is a biomedical institute A which exists to conduct stem cell and related research for Bedford Stem Cell diseases and conditions which currently have no eective Research Foundation methods of treatment or cure. PO Box 1028 Bedford, MA 01730 THE POWER OF PRIVATE FUNDING Please enclose the name The tax-exempt status granted to public charities highlights the and address of anyone you U.S. Government’s belief that taxpayers have the right to directly would like to be notied of support activities they feel are important. To advance the your contribution or added treatment opportunities, to grow its community of scholars, and to our mailing list. to enlarge the potential for miracles, the Foundation needs the Donate Online at: help of private benefactors to raise funds. www.bedfordresearch.org BOARD OF TRUSTEES ETHICS ADVISORY BOARD Alan S. Geismer, Esq Chairman of the Board Arthur Applbaum, PhD Chairman of the Board PARTNER, SUGARMAN, ROGERS, BARSHAK AND COHEN PROF. OF ETHICS AND PUBLIC POLICY, Jose Cibelli, DVM PhD KENNEDY SCHOOL OF GOVERNMENT PROF. OF ANIMAL BIOTECHNOLOGY, MICHIGAN STATE UNIVERSITY Stanley J. Bodner, MD INFECTIOUS DISEASE SPECIALIST Lawrence R. Jones CEO, JONES MARKETING SERVICES Kenneth A. Burry, MD PROF. OF OBSTETRICS AND GYNECOLOGY Robert L. Kaufmann, MD OREGON HEALTH SCIENCES UNIVERSITY REPRODUCTIVE ENDOCRINOLOGIST Norman Daniels, PhD PROF. OF ETHICS & PUBLIC HEALTH, Sean Kealy, Esq BOSTON UNIVERSITY SCHOOL OF LAW HARVARD SCHOOL OF PUBLIC HEALTH John D. -

Assessing Corporate Bioethics a Qualitative Exploration of How
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ASU Digital Repository Assessing Corporate Bioethics A Qualitative Exploration of How Bioethics is Enacted in Biomedicine Companies by Jennifer Brian A Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Approved October 2011 by the Graduate Supervisory Committee: Jason Robert, Chair Daniel Sarewitz James Hurlbut Jonathan Moreno Mark Brown Jane Maienschein ARIZONA STATE UNIVERSITY May 2012 ©2012 Jennifer Elizabeth Dyck Brian All Rights Reserved ABSTRACT Corporations in biomedicine hold significant power and influence, in both political and personal spheres. The decisions these companies make about ethics are critically important, as they help determine what products are developed, how they are developed, how they are promoted, and potentially even how they are regulated. In the last fifteen years, for-profit private companies have been assembling bioethics committees to help resolve dilemmas that require informed deliberation about ethical, legal, scientific, and economic considerations. Private sector bioethics committees represent an important innovation in the governance of emerging technologies, with corporations taking a lead role in deciding what is ethically appropriate or problematic. And yet, we know very little about these committees, including their structures, memberships, mandates, authority, and impact. Drawing on an extensive literature review and qualitative analysis of semi-structured interviews with executives, scientists and board members, this dissertation provides an in-depth analysis of the Ethics and Public Policy Board at SmithKline Beecham, the Ethics Advisory Board at Advanced Cell Technology, and the Bioethics Committee at Eli Lilly and offers insights about how ideas of bioethics and governance are currently imagined and enacted within corporations.