Overwintering of West Nile Virus in a Bird Community with a Communal Crow Roost Received: 19 December 2017 Diego Montecino-Latorre 1 & Christopher M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Snowschool Offered to Local Students Environment
6 TUESDAY, JANUARY 28, 2020 The Inyo Register SnowSchool offered to local students environment. The second with water. The food color- journey is unique. This Bishop, session allows students to ing and glitter represent game shows students how Mammoth review the first lesson and different, pollutants that water moves through the learn how to calculate snow might enter the watershed, earth, oceans, and atmo- Lakes fifth- water equivalent. The final and students can observe sphere, and gives them a grade students session takes students how the pollutants move better understanding of from the classroom to the and collect in different the water cycle. participate in mountains for a SnowSchool bodies of water. For the final in-class field day. Once firmly in For the second in-class activity, students learn SnowSchool snowshoes, the students activity, students focus on about winter ecology and learn about snow science the water cycle by taking how animals adapt for the By John Kelly hands-on and get a chance on the role of a water mol- winter. Using Play-Doh, Education Manager, ESIA to play in the snow. ecule and experiencing its they create fictional ani- During the in-class ses- journey firsthand. Students mals that have their own For the last five years, sion, students participate break up into different sta- winter adaptations. Some the Eastern Sierra in three activities relating tions. Each station repre- creations in past Interpretive Association to watersheds, the water sents a destination a water SnowSchools had skis for (ESIA) and Friends of the cycle, and winter ecology. molecule might end up, feet to move more easily Inyo have provided instruc- In the first activity, stu- such as a lake, river, cloud, on the snow and shovels tors who deliver the Winter dents create their own glacier, ocean, in the for hands for better bur- Wildlands Alliance’s watershed, using tables groundwater, on the soil rowing ability. -

Stars Gather
Veterinary Medical Clinic January 25 - 31, 2020 William Oglesby, DVM We Treat Both Small Animals and Large Animals 804 Southeast Boulevard Clinton, NC 28328 Monday-Friday Stars 7:30am-5:30pm (910) 592-3338 Healthy Animals are gather Happy Animals Alicia Keys hosts the 2020 Grammy Awards AUTO HOME FLOOD LIFE WORK Courtney Bennett Agent [email protected] 919-920-5195 101 E. Clinton St. Roseboro NC We ought to weigh well, SEE WHAT YOUR NEIGHBORS ARE TALKING ABOUT! what we can only once decide. Complete Funeral Service including: Traditional Funerals, Cremation Outdoor Power Equipment Pre-Need-Pre-Planning Independently Owned & Operated Since 1920’s Complete parts Butler Funeral Home and service department! 401 W. Roseboro Street 2 locations to Hwy. 24 Windwood Dr. Roseboro, NC better serve you Stedman, NC 401 NE Blvd., Clinton, NC • 910-592-7077 • www.clintonappliance.com 910-525-5138 910-223-7400 910-525-4337 (fax) 910-307-0353(fax) Page 2 — Saturday, January 25, 2020 — Sampson Independent On the Cover Singing praises: Year’s best artists honored at the 62nd Annual Grammy Awards By Sachi Kameishi TV Media here’s something for everyone Tin the televised ceremonies that populate awards season. From boozy Golden Globes banter to the Emmys’ quirky hosting history and BAFTAs charming decorum, each of the major awards shows has its own way of celebrating its particu- lar craft and making the show re- warding and accessible to its mil- lions of viewers. The ratings vary year to year, and although the Academy Awards tends to walk away from the season as the most- watched awards show, the Gram- my Awards is a close second. -
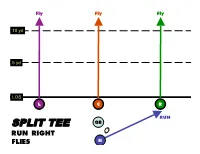
Sample Playbook
Fly Fly Fly 10 yd 5 yd LOS L C R RUN SPLIT TEE QB RUN RIGHT FLIES H Fly Fly Fly 10 yd 5 yd LOS L C R RUN QB SPLIT TEE RUN LEFT H FLIES 10 yd Post Flag 5 yd Curl Circle LOS L C R QB SPLIT TEE CIRCLE F H POST - FLAG - CURL Fly 10 yd 5 yd In Curl Arrow LOS L C R SPLIT TEE QB F ARROW CURL – FLY - OUT H SHORT YARDAGE 10 yd TWO POINT PAT 5 yd In Curl Slant Out In LOS L C R SPLIT TEE QB OUT F IN - CURL - SLANT H IN 10 yd Post Fade Out 5 yd Curl LOS L C R SPLIT TEE QB Flare Flare F Curl - FadeOUT - H FLAG 10 yd Flag 5 yd Out Slant In LOS L C R SPLIT TEE QB OPTION PASS Slant IN - POST - H OPTION PASS OUT 10 yd Fly 5 yd LOS L C R QB SPLIT TEE H RIGHT REVERSE 10 yd Post Fade In 5 yd Curl Curl LOS L H C R QB TWINS LEFT FADE IN - DOUBLE CURL - POST 10 yd In In 5 yd In In LOS L H C R QB TWINS LEFT INs 10 yd Flag Post Fade In 5 yd Slant In LOS L H C R QB TWINS LEFT FADE - SLANT IN – FLAG - POST 10 yd Post Fade Out In 5 yd Curl LOS L H C R QB TWINS LEFT Post – IN - FADE OUT - CURL 10 yd Fade In 5 yd Out Out Slant In LOS L C H R QB TWINS RIGHT INS & OUTS 10 yd Flag Fade Out Fade In 5 yd Slant In LOS L C H R QB TWINS RIGHT FADE OUT - FLAG - FADE IN - SLANT IN Fly Fly 10 yd 5 yd Out Curl LOS L C H R QB TWINS RIGHT BOMB - CURL - BOMB - OUT 10 yd SHORT YARDAGE ONE POINT PAT 5 yd Out In Slant Curl In LOS L C H R QB TWINS RIGHT Slant In – OUT – IN - CURL Out 10 yd Out Out 5 yd Out LOS L H R C QB TRIPS LEFT OUTS 10 yd Flag Flag Cur 5 yd l Curl LOS L H R C QB TRIPS LEFT DEEP & TIGHT 10 yd Slant Slant In 5 yd Out Slant Slant Out In LOS L H R C QB TRIPS LEFT SLANT -

David Ramsey Ç”Μå½± ĸ²È¡Œ (Ť§Å…¨)
David Ramsey 电影 串行 (大全) Dead to Rights https://zh.listvote.com/lists/film/movies/dead-to-rights-16555590/actors Suicide Squad https://zh.listvote.com/lists/film/movies/suicide-squad-16636526/actors The Secret Origin of Felicity https://zh.listvote.com/lists/film/movies/the-secret-origin-of-felicity-smoak-18416478/actors Smoak Legacies https://zh.listvote.com/lists/film/movies/legacies-21075036/actors Invasion! https://zh.listvote.com/lists/film/movies/invasion%21-28518213/actors Legacy https://zh.listvote.com/lists/film/movies/legacy-28517940/actors The Odyssey https://zh.listvote.com/lists/film/movies/the-odyssey-16639693/actors Three Ghosts https://zh.listvote.com/lists/film/movies/three-ghosts-16640101/actors Brotherhood https://zh.listvote.com/lists/film/movies/brotherhood-21484189/actors Lone Gunmen https://zh.listvote.com/lists/film/movies/lone-gunmen-9023621/actors Muse of Fire https://zh.listvote.com/lists/film/movies/muse-of-fire-16609655/actors Kapiushon https://zh.listvote.com/lists/film/movies/kapiushon-30634955/actors Unfinished Business https://zh.listvote.com/lists/film/movies/unfinished-business-16644267/actors Identity https://zh.listvote.com/lists/film/movies/identity-16577364/actors Betrayal https://zh.listvote.com/lists/film/movies/betrayal-17622803/actors Keep Your Enemies Closer https://zh.listvote.com/lists/film/movies/keep-your-enemies-closer-16586458/actors The Calm https://zh.listvote.com/lists/film/movies/the-calm-18285639/actors Vertigo https://zh.listvote.com/lists/film/movies/vertigo-16645902/actors -
BAVPA Beacon Alumni Newsletter
If you would like to unsubscribe The BAVPA Beacon newsletter, please contact RON MANGUM ( [email protected] ) or RUSSELL LINK at ([email protected]). This letter is a great way to keep everyone informed about what’s happening in our lives; please help us to keep it going. LATEST BAVPA ALUMNI NEWS AND UPDATES Actor and Director DANIEL CROIX HENDERSON (2013) appears on the BET Network Tyler Perry's White House political drama, The Oval. The rebroadcast of the episode, The United Front airs January 17at 9PM:https://www.bet.com/shows/tyler-perrys-the-oval.html. Henderson’s official website: https://www.danielcroix.com/work. The GRACE STUMBERG ( 2007) Band performs at Thin Man Brewery, 492 Elmwood Avenue, Buffalo January 17. For late January and February concert dates, go to https://gracestumberg.com/. Educator, Saxophonist and Composer JAMES BRANDON LEWIS (2001) performs at The Rauschenberg Residency in Captiva, FL April 6. http://www.jblewis.com/. Lewis’ CD, An Unruly Manifesto ( https://jamesbrandonlewis.bandcamp.com/releases ), is available for purchase and was nominated for the Gilles Peterson Worldwide Awards’ ( https://worldwidefm.net) album of the year. Celebrity Make Up Artist SIR JOHN BARNETT (2000) created the Luminess Cosmetics Limited Edition /Disney’s The Lion King Make Up Collection:https://www.luminessbeauty.com/disney/Home. InsidetheMagic.com/The Lion King related article, Disney’s 2020 Oscar Nominations: “The Lion King”, “Avengers: Endgame”, and more ( https://insidethemagic.net/2020/01/2020-disney-oscar-nominations-kc1/), Lion King was nominated for Best Visual Effects and the Oscars ( https://abc.com/shows/oscars ) airs February 9 on ABC. -

BOW MAKING with Justin Sutera
BOW MAKING with Justin Sutera PART III 615 Willow Ave., Ithaca, NY 14850 | primitivepursuits.com | 607-272-2292 ext. 195 About the Author Justin Sutera first got interested in primitive living skills when he was 14 after being introduced to ‘The Tracker’ written by Tom Brown. Since that time Justin has explored primitive skills deeply (often by trial and error in the early days). He graduated S.U.N.Y. College of Environmental Science and Forestry with a B.S. in Conservation Biology. While still a student Justin founded ESF Primitive Pursuits Club. Additionally Justin has studied at the Tracker School and worked as an instructor at The Children Of The Earth Foundation before joining Primitive Pursuits as a full time instructor. Justin is particularly passionate about primitive bow (and arrow) making, flintknapping, and hide tanning but truthfully all skills for living with the Earth are of great interest to him. As a lead instructor with Primitive Pursuits, Justin focuses on teen and adult programming including Wilderness Weekends, the Wilderness Skills Intensive, and the Wilderness Year program. About Primitive Pursuits Primitive Pursuits is a non-profit program in partnership with Cornell Cooperative Extension. We provide leadership and wilderness skills education to hundreds of toddlers, youth, teens and adults throughout the Finger Lakes region. Since 2002 it has been our mission to steward the health of our community by fostering life-long relationships with the natural world through exceptional mentoring and nature education. Through our mission, we work daily to achieve a cultural intervention that will bring back into our modern lives a necessary and healthy relationship with the natural world and within our human communities.experience, For more please visit primitivepursuits.com Introduction First of all, I want to mention that there are tons of fantastic books and other resources out there on bow making. -

Ku Klux Klan
If you have issues viewing or accessing this file contact us at NCJRS.gov. KU \ , I, KLUX KLAN A REPORT TO THE ILLINOIS GENERAL ASSEMBLY t;J ~ . ~t ., E J : li,:( {.:.<~ ~,' i .,i i " ,.~ i 1 , .. ,p~~ ; £~C(, .:~.~} ... t <.) ~ '":~~:~ ,~ \ BYTHE ILLINOIS LEGISLATIVE INVESTIGATING COMMISSION 300 West Washington Street, Chicago, Illinois 60606 Telephone (312) 793-2606 ,= OCTOBER 1976 I. Printed bv the Authoritv of the State of Illinois Twenty-Five Hundred Copies TABLE OF CONTENTS HOUSE RESOLUTION 146.................................. iii LETTER TO HONORABLE MEMBERS OF THE GENERAL ASSEMBLY... v INTRODUCTION. • . • . 1 Chapter 1 HISTORICAL OVERVIEW OF THE KU KLUX KLAN A. Introduction.............................. 3 B. Origin of the Ku Klux Klan................ 3 C. Transformation and Growth of the Ku Klux Klan. ... 5 D. Decline of the Klan....................... 10 E. Romanticizing the Klan.................... 11 F. Resurrection of the Ku Klux Klan.......... 16 G. 1950's Klan Revival....................... 23 H. F.B.I. Investigation Breaks the Klan...... 27 I. Present Klan Organizations................ 29 Chapter 2 ORGANIZATION OF THE KU KLUX KLAN A. Introduction.............................. 31 B. Invi sible Empire ......................... It 32 C. Adminis'trative and Command Structure...... 32 D. Purposes of the KKK....................... 35 E. Functions of Local Klans .............. ,... 37 F. A Final Comment on Klan Organization...... 38 Chapter 3 BRIEF HIS~ORY OF THE KLAN IN ILLINOIS A. Introduction.............................. 39 B. The Klan in Illinois during the 1920s..... 39 C. Later Klan Activity....................... 41 Chapter 4 ILLINOIS KLAN'S INITIAL ORGANIZATIONAL ACTIVITY A. Background. 43 B. The Illinois Klan's Early Organizational Efforts.................................... 44 C. Initial Infiltration of the Illinois Klan ...................... ~ . .. .. 45 D. Split in the Illinois Klan Leadership.... -

Analog Audio Recording
The Art of Sound Reproduction This Page Intentionally Left Blank The Art of Sound Reproduction John Watkinson Focal Press An imprint of Butterworth-Heinemann Ltd 225 Wildwood Avenue, Woburn, MA 01801-2041 Linacre House, Jordan Hill, Oxford OX2 8DP A member of the Reed Elsevier plc group OXFORD JOHANNESBURG BOSTON MELBOURNE NEW DELHI SINGAPORE First published 1998 John Watkinson 1998 All rights reserved. No part of this publication may be reproduced in any material form (including photocopying or storing in any medium by electronic means and whether or not transiently or incidentally to some other use of this publication) without the written permission of the copyright holder except in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of a licence issued by the Copyright Licensing Agency Ltd, 90 Tottenham Court Road, London, England W1P 9HE. Applications for the copyright holder’s written permission to reproduce any part of this publication should be addressed to the publishers British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Library of Congress Cataloguing in Publication Data A catalogue record for this book is available from the Library of Congress ISBN 0 240 51512 9 Typeset by Laser Words, Madras, India Printed and bound in Great Britain Contents Preface xiii Chapter 1 Introduction 1 1.1 A short history 1 1.2 Types of reproduction 8 1.3 Sound systems 12 1.4 Portable consumer equipment 14 1.5 Fixed consumer equipment 14 -

SENSUS NAVIGATION Text and Symbols on the Screen Event at Next Guidance Point
SENSUS NAVIGATION Text and symbols on the screen Event at next guidance point Distance to next guidance point Name of next road/street Facility/point of interest (POI) Planned route final destination Planned route intermediate destination Symbol for destination/final destination Calculated arrival time at final destination Calculated remaining distance to destination Compass Traffic information Section covered by traffic information Road type Colour / paint Current road/street name - at car's current position (15) Planned route Motorway Orange The car's current position Primary road Dark grey Stored location Smaller primary road Grey Map scale Ordinary road Light grey Local road White SENSUS NAVIGATION Sensus Navigation is a satellite based traffic information and this supplement differ from the equipment in the car. We reserve the route guidance system. right to make modifications without prior notice. Development work is constantly in progress to improve our product. Modifications may mean that information, descriptions and illustrations in TABLE OF CONTENTS INTRODUCTION SPECIFY DESTINATION, TRAFFIC INFORMATION Introduction 6 ITINERARY AND ROUTE Navigation - traffic information 34 Sensus Navigation 7 Navigation - specify destination 16 Navigation - Real Time Traffic Infor- 36 mation (RTTI), Navigation - operation 10 Navigation - points of interest (POI) 20 symbols Navigation - character wheel and 12 keyboard Navigation - itinerary 21 Navigation - voice recognition 13 Navigation - route 22 Navigation - route options 24 Navigation - map options 27 Navigation - guidance options 29 Navigation - importing/exporting 31 stored locations 2 MAP AND SYSTEM INFORMATION ALPHABETICAL INDEX Navigation - map and system information 40 Alphabetical Index 49 Navigation - menu overview 41 Navigation - fault tracing 44 Navigation - license agreement and 46 copyright 3 INTRODUCTION INTRODUCTION Introduction Owner's Manual in mobile devices This supplement is a supplement to the normal owner's manual for the car. -
World Archery, Glossary of Terms
World Archery Coaching Manual for entry level away from the archer, when holding up the bow. Glossary of Terms: Bare shaft: An arrow shaft without fletchings. Actual draw length: The personal draw length Bare-bow: A class of shooting where the bow has a single string and the bow is held with one of the archer measured at full draw, from the hand and the string is drawn and released with bottom of the slot in the nock to the pivot point the fingers of the other hand. No sighting marks of the grip plus 1 3/4 inch, which is on most of or protruding stabilisers are permitted. the bow the back edge (far side) of the bow. Barrelled arrow: An arrow that has a greater Actual arrow length: The personal arrow cross section in the middle and tapers down at length of the archer, measured from the bottom both ends. slot of the nock to the end of the shaft (this measurement does not include the pile); with Basic technique: The fundamental technique of this end of the shaft at 1 inch in front of the shooting a bow and arrow. Usually the style vertical passing by the deepest point of the bow taught during the introduction to archery, grip. forming the basis for consistent shooting. Actual draw weight: The energy required to Bast: This is a coil of twisted straw that is draw the bow to the actual draw length behind the target face and to which the face is (measured in pounds). attached. Anchor point: A location on the archer's face to Belly (of bow): The surface of the bow facing where the string hand comes to rest at full draw, the archer during shooting. -

Ìœœë¼ Í™€Ëžœë“Œ ̘͙” ˪…˶€ (Ìž'í'ˆìœ¼ë¡Œ)
ìœŒë¼ í™€ëžœë“œ ì˜í ™” 명부 (작품으로) The Secret Origin of https://ko.listvote.com/lists/film/movies/the-secret-origin-of-felicity-smoak-18416478/actors Felicity Smoak Legacies https://ko.listvote.com/lists/film/movies/legacies-21075036/actors Invasion! https://ko.listvote.com/lists/film/movies/invasion%21-28518213/actors Legacy https://ko.listvote.com/lists/film/movies/legacy-28517940/actors The Odyssey https://ko.listvote.com/lists/film/movies/the-odyssey-16639693/actors Three Ghosts https://ko.listvote.com/lists/film/movies/three-ghosts-16640101/actors Brotherhood https://ko.listvote.com/lists/film/movies/brotherhood-21484189/actors Lone Gunmen https://ko.listvote.com/lists/film/movies/lone-gunmen-9023621/actors Muse of Fire https://ko.listvote.com/lists/film/movies/muse-of-fire-16609655/actors Identity https://ko.listvote.com/lists/film/movies/identity-16577364/actors Betrayal https://ko.listvote.com/lists/film/movies/betrayal-17622803/actors Keep Your Enemies https://ko.listvote.com/lists/film/movies/keep-your-enemies-closer-16586458/actors Closer Vertigo https://ko.listvote.com/lists/film/movies/vertigo-16645902/actors Crucible https://ko.listvote.com/lists/film/movies/crucible-16553793/actors Birds of Prey https://ko.listvote.com/lists/film/movies/birds-of-prey-15993780/actors Darkness on the Edge of https://ko.listvote.com/lists/film/movies/darkness-on-the-edge-of-town-16555341/actors Town An Innocent Man https://ko.listvote.com/lists/film/movies/an-innocent-man-8197992/actors Tremors https://ko.listvote.com/lists/film/movies/tremors-16642454/actors -

January 9-19
If you would like to unsubscribe The BAVPA Beacon newsletter, please contact RON MANGUM ( [email protected] ) or RUSSELL LINK at ([email protected]). This letter is a great way to keep everyone informed about what’s happening in our lives; please help us to keep it going. LATEST BAVPA ALUMNI NEWS AND UPDATES GRACE STUMBERG (2007) Band performs at Remington Tavern & Seafood Exchange, 184 Sweeney St, North Tonawanda, NY November 21 at 6:30PM. For late November and December performance dates, go to https://gracestumberg.com/. Educator, Saxophonist and Composer JAMES BRANDON LEWIS (2001) performs at Winter JazzFest ( https://www.winterjazzfest.com/tickets- 4 ) January 9-19. Lewis received the ASCAP Foundation Fred Ho Award. The award founded by Ho http://www.jblewis.com/. Lewis’ CD, An Unruly Manifesto ( https://jamesbrandonlewis.bandcamp.com/releases ), is available for purchase. Celebrity Make Up Artist SIR JOHN BARNETT(2000) created the Luminess Cosmetics Limited Edition /Disney’s The Lion King Make Up Collection; https://www.luminessbeauty.com/disney/Home. InsidetheMagic.com/The Lion King related article, Disney’s live-action “The Lion King” finally gets official Disney+ release date ( https://insidethemagic.net/2019/12/disney-live-action-the-lion-king-disney-plus-kc1/), the will be available on Disney+ January 28. CartoonBrew.com article, Golden Globes Nominees: Disney Dominates, ‘Lion King’ Finally Acknowledged As Animation:https://www.cartoonbrew.com/awards/golden-globes-nominees-disney-dominates-netflix-and-foreign-films- miss-out-183524.html. VICTORIA PEREZ (1997) is the Co-Founder and Artistic Director of Raices Theatre Company (www.raicestheatrecompany.com), SMIRNA MERCEDES-PEREZ (1992) and LISETTE DEJESUS are RTC members; the theatre ensemble is celebrating its sixth anniversary.