H Organometallic Catalysis in Industry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4 Experimental
Chapter 1 Introduction Introduction Chapter 1 1.1 Transition Metal Complexes as Homogeneous Catalysts A variety of transition metals and metal complexes act as catalysts for organic reactions. The use of transition metal complexes to catalyse organic reactions is one of the most important applications of organometallic chemistry and has been the driving force in the rapid development of this field since catalysts are crucial to the turnover and selectivity in many chemical syntheses.1* Organometallic complexes catalyse a wide range of reactions, both in nature and in industry. For example, the naturally occurring metalloenzymes are vital catalysts in many metabolic processes such as respiration and are crucial in the photosynthetic cycle. Transition metal catalysts are used industrially in the production of bulk materials, pharmaceuticals, agrochemicals, flavours and fragrances2 by technological application such as; the hydroformylation of alkenes, the oxidation of alkenes, hydrocyanation of butadiene, and hydrogenation (Scheme 1.1).3 Hydroformylation of Alkenes (Oxo Process) O O H C Co(I) or Rh(I) C R + H2 +CO R H + R * Oxidation of alkenes (Wacker Process) O Pd(II) + Cu(II) + 1 O H 2 2 Hydrocyanation of Butadiene Ni(0) CN + 2HCN NC Hydrogenation (Wilkinson’s Catalyst) [RhCl(PPh3)3] R + H2 R Scheme 0.1 * References begin on page 16. 1 Introduction Chapter 1 One of the most important examples of homogeneous catalysis is the carbonylation of methanol to produce acetic acid, in what is known as the Monsanto Process (Scheme 1.2). Acetic acid is used for different purposes in the industry such as producing vinegar, food preservatives, solvents and plastics. -

Bimetallic Catalyst Catalyzed Carbonylation of Methanol to Acetic Acid
materials Article Study on Rh(I)/Ru(III) Bimetallic Catalyst Catalyzed Carbonylation of Methanol to Acetic Acid Shasha Zhang 1, Wenxin Ji 1,2,*, Ning Feng 2, Liping Lan 1, Yuanyuan Li 1,2 and Yulong Ma 1,2 1 College of Chemistry and Chemical Engineering, Ningxia University, Yinchuan 750021, China; [email protected] (S.Z.); [email protected] (L.L.); [email protected] (Y.L.); [email protected] (Y.M.) 2 State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering, Ningxia University, Yinchuan 750021, China; [email protected] * Correspondence: [email protected]; Tel.: +86-135-1957-9989; Fax: +86-951-206-2323 Received: 13 July 2020; Accepted: 3 September 2020; Published: 11 September 2020 Abstract: In this study, a Rh(I)/Ru(III) catalyst with a bimetallic space structure was designed and synthesized. The interaction between the metals of the bimetallic catalyst and the structure of the bridged dimer can effectively reduce the steric hindrance effect and help speed up the reaction rate while ensuring the stability of the catalyst. X-ray photoelectron spectroscopy (XPS) results show that rhodium accepts electrons from chlorine, thereby increasing the electron-rich nature of rhodium and improving the catalytic activity. This promotes the nucleophilic reaction of the catalyst with methyl iodide and reduces the reaction energy barrier. The methanol carbonylation performance of the Rh/Ru catalyst was evaluated, and the results show that the conversion rate of methyl acetate and the yield of acetic acid are 96.0% under certain conditions. Furthermore, during the catalysis, no precipitate is formed and the amount of water is greatly reduced. -

Oxidative Addition & Reductive Elimination
3/1/2021 Oxidative Addition & Reductive Elimination Robert H. Crabtree: Pages 159 – 182 and 235 - 266 1 Oxidative Addition Change in Example Group configuration • Oxidative addition is a key step in many transition-metal catalyzed reactions 10 8 I III • Basic reaction: d d Cu Cu 11 X X Pd0 PdII 10 LnM + LnM Pt0 PtII 10 Y Y d8 d6 PdII PdIV 10 • The new M-X and M-Y bonds are formed using the electron pair of the X-Y PtII PtIV 10 bond and one metal-centered lone pair IrI IrIII 9 RhI RhIII 9 • The metal goes up in oxidation state (+2), X-Y formally gets reduced to X-, Y- d6 d4 ReI ReIII 7 • The ease of addition (or elimination) can be tuned by the electronic and 0 II steric properties of the ancillary ligands Mo Mo 6 • OA favored by strongly e-donating L d4 d2 MoII MoIV 6 • The most common applications involve: a) Late transition metals (platinum metals: Ru, Rh, Pd, Ir, Pt) - not d7 d6 2CoII 2CoIII 9 too sensitive to O2 and H2O; routinely used in organic synthesis b) C-Halogen, H-H or Si-H bonds d4 d3 2CrII 2CrIII 6 • Common for transition metals, rare for main-group metals but: Grignard reagents! 2 1 3/1/2021 Oxidative addition – concerted mechanism Concerted addition - mostly with non-polar X-Y bonds X X X LnM + LnM LnM Y Y Y A Cr(CO)5: • H2, silanes, alkanes, ... coordinatively Cr(PMe3)5: • Arene C-H bonds more reactive than alkane C-H bonds unsaturated, but metal- centered lone pairs not phosphines are better • H-H, C-H strong bond, but M-H and M-C bonds can be very available donors, weaker acceptors full oxidative -

Wacker Oxidation ~Anti-Markovnikov~
Anti-Markovnikov Olefin Functionalization ~Prof. Robert H. Grubbs’ Work~ 4th Literature Seminar July 5, 2014 Soichi Ito (D1) Contents 1. Introduction • Flow of Prof. Grubbs’ Research • Markovnikov’s Rule • Wacker Oxidation 2. Grubbs’ Work • Substrate-Controlled Wacker Oxidation • Catalyst-Controlled Wacker-Type Oxidation 2 Introduction ~Flow of Research~ Olefin Metathesis Anti-Markovnikov Wacker Oxidation of Terminal Olefin Substrate-Controlled Wacker Oxidation of Internal Olefin Z-Selective Metathesis Hydration Ethenolysis + Reduction Hydroamination Z-Selective Ethenolysis Catalyst-Controlled Decarbonylative Dehydration Hydrophosphonation Production of Terminal Olefin Functionalization of Terminal Olefin 3 Introduction ~Markovnikov’s Rule~ Two-Step Two-Step (+1C) 4 Robert H. Grubbs et al. Science, 2011, 333, 1609. Anti-Markovnikov Hydration of Olefins • One-Step William C. Trogler et al. Science 1986, 233, 1069. This work was difficult to reproduce. Inorg. Chem. 1988, 27, 3151. • One-Step with Activated Olefins Robert G. Bergman and F. Dean Toste et al. J. Am. Chem. Soc. 2003, 125, 8696. Ben L. Feringa and Gerard Roelfes et al. Nat. Chem. 2010, 2, 991. • Three-Step 5 Shannon S. Stahl et al. J. Am. Chem. Soc. 2010, 132, 15116. Anti-Markovnikov Wacker Oxidation / Reduction Strategy Oxidation cycle must be compatible with the reduction cycle. aldehyde-selective Wacker Oxidation 6 Robert H. Grubbs et al. Science, 2011, 333, 1609. Introduction ~Wacker-Tsuji Oxidation~ • 1894 F. C. Phillips reported stoichiometric reaction. • 1959 J. Smidt et al. reported the Wacker process. (oxidation of ethylene to acetaldehyde) Investigations for convenient laboratory methods • 1976 J. Tsuji et al. reported PdCl2, CuCl / DMF, H2O method. “Terminal alkenes may be viewed as masked ketones.” 7 Jacques Muzart Tetrahedron 2007, 63, 7505. -

Development and Application of New Solid-Supported Bis (Oxazolines) In
Development and Application of New Solid-Supported Bis(oxazolines) in the Area of Asymmetric Catalysis INAUGURALDISSERTATION zur Erlangung des Doktorgrades der Fakultät für Chemie, Pharmazie und Geowissenschaften der Albert-Ludwigs-Universität Freiburg im Breisgau vorgelegt von Claudia Stork aus Freiburg April 2009 Die vorliegende Arbeit wurde am Institut für Organische Chemie und Biochemie der Albert- Ludwigs-Universität Freiburg in der Zeit von Mai 2006 bis April 2009 im Arbeitskreis von Herrn Prof. Dr. W. Bannwarth angefertigt. Vorsitzender des Promotionsausschusses: Prof. Dr. R. Schubert Referent: Prof. Dr. W. Bannwarth Koreferent: Prof. Dr. Ch. Janiak Datum der Promotion: 05.11.2009 Danksagung Herrn Prof. Dr. Bannwarth danke ich für die interessante Aufgabenstellung, die gewährten Freiheiten bei der Ausgestaltung des Themas sowie die großzügige Unterstützung. DSM danke ich für die finanzielle Unterstützung der vorliegenden Arbeit. Herrn Prof. Dr. Bonrath (DSM) möchte ich für die wertvollen Diskussionen und die gute Zusammenarbeit danken. Für die freundliche Übernahme des Koreferats danke ich Herrn Prof. Dr. Janiak. Allen ehemaligen und gegenwärtigen Mitarbeitern unserer Arbeitsgruppe danke ich für die ständige Diskussionsbereitschaft, die angenehme Arbeitsatmosphäre und die Hilfsbereitschaft. Namentlich genannt seinen dabei vor allem: Dominik Altevogt, Luigi Rumi, Rolf Kramer und Hartmut Rapp. Für das sorgfältige Korrekturlesen dieser Arbeit danke ich Jan-Frederik Blank, Luigi Rumi und Dominik Altevogt. Bei Fr. Hirth-Walter bedanke ich mich für die Durchführung der AAS-Messungen. Herrn Dr. Harald Scherer danke ich für die Aufnahme der HR-MAS-NMR Spektren. Selbstverständlich sei auch allen Mitgliedern der Service-Abteilungen gedankt. Besonders hervorheben möchte ich Herrn Fehrenbach für die zahlreichen HPLC-Messungen. Mein größter Dank geht jedoch an meine Familie und Jan-Frederik für ihre Geduld und ihre moralische Unterstützung. -

New Reactions of Cyclic Oxygen, Nitrogen and Sulfur Acetal Derivatives
New Reactions of Cyclic Oxygen, Nitrogen and Sulfur Acetal Derivatives Samuel Edward Mann Supervisor Dr. Tom Sheppard A thesis submitted to University College London in partial fulfilment of the requirements for the degree of Doctor of Philosophy Declaration I, Samuel Edward Mann confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. ………………………… i Abstract Abstract This thesis describes the development of new reactions of cyclic oxygen, nitrogen and sulfur acetal derivatives and their applications in a diverse range of synthetic organic and organometallic chemistry. Detailed herein are advances in three main areas of acetal chemistry, namely: studies towards a new methodology for the synthesis of medium ring heterocycles; the use of thioacetals as directing groups for the palladium-mediated oxidation of olefins; and multi-component reactions for the synthesis of drug-like heterocyclic compounds. A brief overview of the chemistry of cyclic acetal derivatives is given in the first chapter, followed by a chapter on each of the three areas investigated. Relevant introductory literature is reviewed at the beginning of each chapter. Firstly, the ring expansion chemistry of unsaturated cyclic oxygen, nitrogen and sulfur acetal derivatives was explored for the development of a new methodology for the synthesis of medium ring heterocycles. This methodology has thus far proved unsuccessful in the synthesis of medium rings, although several interesting and unusual transformations were observed, such as the unexpected formation of an intriguing bicyclic enaminium salt. The use of thioacetals as directing groups for the palladium-mediated oxidation of terminal olefins was also explored, leading to the evolution of a new methodology for the catalytic, regioselective formation of either vinyl or allylic acetates. -

Organic Seminar Abstracts
Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/organicsemin1971752univ / a ORGANIC SEMINAR ABSTRACTS 1971-1972 Semester II School of Chemical Sciences Department of Chemistry- University of Illinois Urbana, Illinois 3 SEMINAR TOPICS II Semester 1971-72 Reactions of Alkyl Ethers Involving n- Complexes on a Reaction Pathway 125 Jerome T. Adams New Syntheses of Helicenes 127 Alan Morrice Recent Advances in Drug Detection and Analysis I36 Ronald J. Convers The Structure of Carbonium Ions with Multiple Neighboring Groups 138 William J. Work Recent Reactions of the DMSO-DCC Reagent ll+O James A. Franz Nucleophilic Acylation 1^2 Janet Ollinger The Chemistry of Camptothecin lU^ Dale Pigott Stereoselective Syntheses and Absolute Configuration of Cecropia Juvenile Horomone 1U6 John C. Greenfield Uleine and Related Aspidosperma Alkaloids 155 Glen Tolman Strategies in Oligonucleotide Synthesis 162 Graham Walker Stable Carbocations: C-13 Nuclear Magnetic Resonance Studies 16U Moses W. McMillan Organic Chlorosulfinates 166 Steven W. Moje Recent Advances in the Chemistry of Penicillins and Cephalosporins 168 Ronald Stroshane Cerium (iv) Oxidations 175 William C. Christopfel A New Total Synthesis of Vitamin D 18 William Graham Ketone Transpositions 190 Ann L. Crumrine - 125 - REACTIONS OF ALKYL ETHERS INVOLVING n-COMPLEXES ON A REACTION PATHWAY Reported by Jerome T. Adams February 2k 1972 The n-complex (l) has been described as an intermediate on the reaction pathway for electrophilic aromatic substitution and acid catalyzed rearrange- ment of alkyl aryl ethers along with sigma (2) and pi (3) type intermediates. 1 ' 2 +xR Physical evidence for the existence of n-complexes of alkyl aryl ethers was found in the observation of methyl phenyl ononium ions by nmr 3 and ir observation of n-complexes of anisole with phenol. -

Manipulating Selectivity and Reactivity in Palladium-Catalyzed Oxidation Reactions
Manipulating Selectivity and Reactivity in Palladium-Catalyzed Oxidation Reactions Thesis by Zachary Kimble Wickens In Partial Fulfillment of the Requirements for the degree of Doctor of Philosophy CALIFORNIA INSTITUTE OF TECHNOLOGY Pasadena, California 2015 (Defended June 2nd, 2015) © 2015 Zachary Kimble Wickens All Rights Reserved 1 Dedicated to my parents for never pushing me to become a scientist 2 Acknowledgments The work I have the pleasure of sharing with you in this thesis would have been completely impossible without the help (both emotional and intellectual) of a tremendously large number of people. First, I want to thank my advisor, Bob Grubbs. I truly could not have asked for a more incredible thesis advisor. Since the beginning of my time at Caltech, you have subtly guided me towards the water but never forced me to drink. You have given me independence in choosing which projects to pursue and the directions these projects go, but always provided suggestions and hints along the way that have kept me on track. I know you will continue to provide great advice and strong support even long after I've left Caltech. Thanks for being my invisible safety net over the last five years. I would have never even considered pursuing a Ph.D in chemistry in the first place if it weren't for the fantastic chemistry faculty at Macalester College. Rebecca Hoye, Ronald Brisbois, Paul Fischer, Keith Kuwata, Thomas Varberg and Katherine Splan, you were all tremendously influential in my life. You taught me how to see the exciting puzzles that are scattered throughout the field of chemistry. -

Novel Co-Mo/MCM-41 Catalysts for Deep Hydrodesulfurization of Jet Fuel
Elucidating the mechanism of heterogeneous Wacker oxidation over response from Pd speciation (Figure 1) demonstrating for the first time that oxygen is activated Pd-Cu-exchanged zeolite Y via transient multi-edge XAS on copper in the heterogeneous system, which is similar to the homogeneous system. Increasing the O partial pressure led to a quick reoxidation of Cu(I) with a concomitant rapid 2 increase in CO formation while that of acetaldehyde proceeded more slowly. These results Jerick Imbao1,2, Jeroen van Bokhoven1,2*, Adam Clark1 and Maarten Nachtegaal1* 2 indicate that copper has more than one role as it does not only act as a co-catalyst for Pd(0) 1Paul Scherrer Institute, Villigen PSI (Switzerland) regeneration, but is also involved in the formation of CO byproduct. Without detecting the 2ETH Zurich, Zurich (Switzerland) 2 transient presence of Cu(0) and Pd(I), our results suggest that two one-electron transfers from *[email protected], [email protected] two Cu(II) ions to reoxidize Pd(0) to Pd(II) is at work in this heterogeneous Wacker catalyst. Introduction Low O (half order) High O (zero order) The homogeneous ethylene oxidation, called the Wacker process, is one of the most 1.00 2 1.00 2 efficient synthetic methods for manufacturing aldehydes. This process suffers from the difficulty in the separation of the products from the catalyst and from the high corrosivity and 0.75 0.75 formation of chlorinated byproducts associated with the excess use of HCl [1]. To overcome Cu(II) Cu(II) these problems many studies have been undertaken to design chloride-free heterogeneous 0.50 0.50 Cu(I) Cu(I) Wacker catalyst systems. -

Metal Carbonyls
MODULE 1: METAL CARBONYLS Key words: Carbon monoxide; transition metal complexes; ligand substitution reactions; mononuclear carbonyls; dinuclear carbonyls; polynuclear carbonyls; catalytic activity; Monsanto process; Collman’s reagent; effective atomic number; 18-electron rule V. D. Bhatt / Selected topics in coordination chemistry / 2 MODULE 1: METAL CARBONYLS LECTURE #1 1. INTRODUCTION: Justus von Liebig attempted initial experiments on reaction of carbon monoxide with metals in 1834. However, it was demonstrated later that the compound he claimed to be potassium carbonyl was not a metal carbonyl at all. After the synthesis of [PtCl2(CO)2] and [PtCl2(CO)]2 reported by Schutzenberger (1868) followed by [Ni(CO)4] reported by Mond et al (1890), Hieber prepared numerous compounds containing metal and carbon monoxide. Compounds having at least one bond between carbon and metal are known as organometallic compounds. Metal carbonyls are the transition metal complexes of carbon monoxide containing metal-carbon bond. Lone pair of electrons are available on both carbon and oxygen atoms of carbon monoxide ligand. However, as the carbon atoms donate electrons to the metal, these complexes are named as carbonyls. A variety of such complexes such as mono nuclear, poly nuclear, homoleptic and mixed ligand are known. These compounds are widely studied due to industrial importance, catalytic properties and structural interest. V. D. Bhatt / Selected topics in coordination chemistry / 3 Carbon monoxide is one of the most important π- acceptor ligand. Because of its π- acidity, carbon monoxide can stabilize zero formal oxidation state of metals in carbonyl complexes. 2. SYNTHESIS OF METAL CARBONYLS Following are some of the general methods of preparation of metal carbonyls. -

Rh(I) Complexes in Catalysis: a Five-Year Trend
molecules Review Rh(I) Complexes in Catalysis: A Five-Year Trend Serenella Medici * , Massimiliano Peana * , Alessio Pelucelli and Maria Antonietta Zoroddu Department of Chemistry and Pharmacy, University of Sassari, Vienna 2, 07100 Sassari, Italy; [email protected] (A.P.); [email protected] (M.A.Z.) * Correspondence: [email protected] (S.M.); [email protected] (M.P.) Abstract: Rhodium is one of the most used metals in catalysis both in laboratory reactions and industrial processes. Despite the extensive exploration on “classical” ligands carried out during the past decades in the field of rhodium-catalyzed reactions, such as phosphines, and other com- mon types of ligands including N-heterocyclic carbenes, ferrocenes, cyclopentadienyl anion and pentamethylcyclopentadienyl derivatives, etc., there is still lively research activity on this topic, with considerable efforts being made toward the synthesis of new preformed rhodium catalysts that can be both efficient and selective. Although the “golden age” of homogeneous catalysis might seem over, there is still plenty of room for improvement, especially from the point of view of a more sustainable chemistry. In this review, temporally restricted to the analysis of literature during the past five years (2015–2020), the latest findings and trends in the synthesis and applications of Rh(I) complexes to catalysis will be presented. From the analysis of the most recent literature, it seems clear that rhodium-catalyzed processes still represent a stimulating challenge for the metalloorganic chemist that is far from being over. Keywords: rhodium; catalysis; Rh(I) complexes Citation: Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. Rh(I) Complexes in Catalysis: A Five-Year 1. -
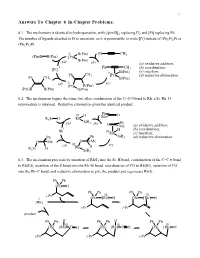
Answers to Chapter 6 In-Chapter Problems
1 Answers To Chapter 6 In-Chapter Problems. 6.1. The mechanism is identical to hydrogenation, with [(pin)B]2 replacing H2 and [Pt] replacing Pd. The number of ligands attached to Pt is uncertain, so it is permissible to write [Pt] instead of (Ph3P)2Pt or (Ph3P)3Pt. II B(Pin) Ph CH3 (Pin)B B(Pin) [Pt] B(Pin) (a) (b) (a) oxidative addition; 0 Ph CH (b) coordination; [Pt] 3 II B(Pin) (c) insertion; Ph CH3 [Pt] (d) reductive elimination. Ph CH3 B(Pin) (d) II (c) [Pt] B(Pin) (Pin)B B(Pin) B(Pin) 6.2. The mechanism begins the same, but after coordination of the C=O π bond to Rh, a Si–Rh–O intermediate is obtained. Reductive elimination gives the identical product. Ph III H Me O R3Si H Rh (a) SiR3 (b) Ph O Me (a) oxidative addition; I H (b) coordination; Rh IIIRh (c) insertion; Ph Ph SiR3 (d) reductive elimination. O Me O Me III (c) (d) Rh H R3Si H SiR3 6.3. The mechanism proceeds by insertion of Rh(I) into the Si–H bond, coordination of the C=C π bond to Rh(III), insertion of the π bond into the Rh–Si bond, coordination of CO to Rh(III), insertion of CO into the Rh–C bond, and reductive elimination to give the product and regenerate Rh(I). Ph Ph OSi H Ph Ph Ph Ph i-Pr III III I OSi [Rh] H OSi [Rh] H [Rh] i-Pr i-Pr product Ph Ph H Ph Ph H Ph Ph III III III OSi [Rh] C O OSi [Rh] C O OSi [Rh] H i-Pr i-Pr i-Pr Chapter 6 2 6.4.