Morphology of the Peritoneal Cavity and Pathophysiological Consequences
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bariatric Surgery in Adolescents: to Do Or Not to Do?
children Review Bariatric Surgery in Adolescents: To Do or Not to Do? Valeria Calcaterra 1,2 , Hellas Cena 3,4 , Gloria Pelizzo 5,*, Debora Porri 3,4 , Corrado Regalbuto 6, Federica Vinci 6, Francesca Destro 5, Elettra Vestri 5, Elvira Verduci 2,7 , Alessandra Bosetti 2, Gianvincenzo Zuccotti 2,8 and Fatima Cody Stanford 9 1 Pediatric and Adolescent Unit, Department of Internal Medicine, University of Pavia, 27100 Pavia, Italy; [email protected] 2 Pediatric Department, “V. Buzzi” Children’s Hospital, 20154 Milan, Italy; [email protected] (E.V.); [email protected] (A.B.); [email protected] (G.Z.) 3 Clinical Nutrition and Dietetics Service, Unit of Internal Medicine and Endocrinology, ICS Maugeri IRCCS, 27100 Pavia, Italy; [email protected] (H.C.); [email protected] (D.P.) 4 Laboratory of Dietetics and Clinical Nutrition, Department of Public Health, Experimental and Forensic Medicine, University of Pavia, 27100 Pavia, Italy 5 Pediatric Surgery Department, “V. Buzzi” Children’s Hospital, 20154 Milan, Italy; [email protected] (F.D.); [email protected] (E.V.) 6 Pediatric Unit, Fond. IRCCS Policlinico S. Matteo and University of Pavia, 27100 Pavia, Italy; [email protected] (C.R.); [email protected] (F.V.) 7 Department of Health Sciences, University of Milan, 20146 Milan, Italy 8 “L. Sacco” Department of Biomedical and Clinical Science, University of Milan, 20146 Milan, Italy 9 Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA; [email protected] * Correspondence: [email protected] Abstract: Pediatric obesity is a multifaceted disease that can impact physical and mental health. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -
The Subperitoneal Space and Peritoneal Cavity: Basic Concepts Harpreet K
ª The Author(s) 2015. This article is published with Abdom Imaging (2015) 40:2710–2722 Abdominal open access at Springerlink.com DOI: 10.1007/s00261-015-0429-5 Published online: 26 May 2015 Imaging The subperitoneal space and peritoneal cavity: basic concepts Harpreet K. Pannu,1 Michael Oliphant2 1Department of Radiology, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA 2Department of Radiology, Wake Forest University School of Medicine, Winston-Salem, NC, USA Abstract The peritoneum is analogous to the pleura which has a visceral layer covering lung and a parietal layer lining the The subperitoneal space and peritoneal cavity are two thoracic cavity. Similar to the pleural cavity, the peri- mutually exclusive spaces that are separated by the toneal cavity is visualized on imaging if it is abnormally peritoneum. Each is a single continuous space with in- distended by fluid, gas, or masses. terconnected regions. Disease can spread either within the subperitoneal space or within the peritoneal cavity to Location of the abdominal and pelvic organs distant sites in the abdomen and pelvis via these inter- connecting pathways. Disease can also cross the peri- There are two spaces in the abdomen and pelvis, the toneum to spread from the subperitoneal space to the peritoneal cavity (a potential space) and the subperi- peritoneal cavity or vice versa. toneal space, and these are separated by the peritoneum (Fig. 1). Regardless of the complexity of development in Key words: Subperitoneal space—Peritoneal the embryo, the subperitoneal space and the peritoneal cavity—Anatomy cavity remain separated from each other, and each re- mains a single continuous space (Figs. -

Abdominal Cavity.Pptx
UNIVERSITY OF BABYLON HAMMURABI MEDICAL COLLEGE GASTROINTESTINAL TRACT S4-PHASE 1 2018-2019 Lect.2/session 3 Dr. Suhad KahduM Al-Sadoon F. I . B. M . S (S ur g. ) , M.B.Ch.B. [email protected] The Peritoneal Cavity & Disposition of the Viscera objectives u describe and recognise the general appearance and disposition of the major abdominal viscera • explain the peritoneal cavity and structure of the peritoneum • describe the surface anatomy of the abdominal wall and the markers of the abdominal viscera u describe the surface regions of the abdominal wall and the planes which define them § describe the structure and relations of : o supracolic and infracolic compartments o the greater and lesser omentum, transverse mesocolon o lesser and greater sac, the location of the subphrenic spaces (especially the right posterior subphrenic recess) The abdominal cavity The abdomen is the part of the trunk between the thorax and the pelvis. The abdominal wall encloses the abdominal cavity, containing the peritoneal cavity and housing Most of the organs (viscera) of the alimentary system and part of the urogenital system. The Abdomen --General Description u Abdominal viscera are either suspended in the peritoneal cavity by mesenteries or are positioned between the cavity and the musculoskeletal wall Peritoneal Cavity – Basic AnatoMical Concepts The abdominal viscera are contained either within a serous membrane– lined cavity called the Abdominopelvic cavity. The walls of the abdominopelvic cavity are lined by parietal peritoneum AbdoMinal viscera include : major components of the Gastrointestinal system(abdominal part of the oesophagus, stomach, small & large intestines, liver, pancreas and gall bladder), the spleen, components of the urinary system (kidneys & ureters),the suprarenal glands & major neurovascular structures. -

Carcinomatous Cirrhosis of the Liver with Sarcomatosis of the Peritoneum 1
CARCINOMATOUS CIRRHOSIS OF THE LIVER WITH SARCOMATOSIS OF THE PERITONEUM 1 S. SANES, M.D., AND K. TERPLAN, M.D. (From tile Pathological Laboratory of the Buffalo General Hospital and School of Medicine, University of Buffalo) The following case is reported because of the occurrence of two different types of malignant neoplasm with typical portal cirrhosis of the liver. That a pathogenetic relationship exists between Laennec's cirrhosis and primary carcinoma of the liver is generally recognized. Whether the association of a peritoneal sarcoma with the cirrhosis in this case was more than a coincidence seemed an interesting point for discussion. REPORT OF CASE E. G., 11 white Italian male fifty-seven years old, was admitted to the Buffalo General Hospital on the service of Drs. N. G. Russell and A. H. Aaron, Nov. 25, 1934. He died Nov. 29, 1934. All his adult life he had partaken of large amounts of wine and whiskey daily. At the age of seventeen years he had suffered an attack of jaundice of several weeks' duration. The patient first began to lose weight and strength in 1932 and noticed that his skin was becoming dark. In March 1934 he complained of cramp-like abdominal pain, diarrhea, and bloating. The stools were watery. There was no nausea or vomiting. Upon hos pitalization, April 9, 1934, physical examination revealed that the pupils reacted to light and accommodation. The chest was emphysematous; breath sounds were diminished in both bases. The heart was regular; a systolic murmur was heard. The blood pressure was 118/70. The liver and spleen were palpable three finger breadths below the costal margin. -

7) Anatomy of OMENTUM
OMENTUM ANATOMY DEPARTMENT DR.SANAA AL-SHAARAWY Dr. Essam Eldin Salama OBJECTIVES • At the end of the lecture the students must know: • Brief knowledge about peritoneum as a thin serous membrane and its main parts; parietal and visceral. • The peritonial cavity and its parts the greater sac and the lesser sac (Omental bursa). • The peritoneal folds : omenta, mesenteries, and ligaments. • The omentum, as one of the peritonial folds • The greater omentum, its boundaries, and contents. • The lesser omentum, its boundaries, and contents. • The omental bursa, its boundaries. • The Epiploic foramen, its boundaries. • Mesentery of the small intestine, and ligaments of the liver. • Nerve supply of the peritoneum. • Clinical points. The peritoneum vIs a thin serous membrane, §Lining the wall of the abdominal and pelvic cavities, (the parietal peritoneum). §Covering the existing organs, (the visceral peritoneum). §The potential space between the two layers is the peritoneal cavity. Parietal Visceral The peritoneal Cavity vThe peritoneal cavity is the largest one in the body. vDivisions of the peritoneal cavity : §Greater sac; extends from Lesser Sac diaphragm down to the pelvis. §Lesser sac; lies behind the stomach. §Both cavities are interconnected through the epiploic foramen. §In male : the peritoneum is a closed sac . §In female : the sac is not completely closed because it Greater Sac communicates with the exterior through the uterine tubes, uterus and vagina. The peritoneum qIntraperitoneal and Intraperitoneal viscera retroperitoneal organs; describe the relationship between various organs and their peritoneal covering; §Intraperitonial structure; which is nearly totally covered by visceral peritoneum. §Retroperitonial structure; lies behind the peritoneum, and partially covered by visceral peritoneum. -

BODY CAVITIES and MESENTERY
73: BODY CAVITIES and MESENTERY We've already mentioned that all the organs in the body are wrapped in "bags" made of thin layers of connective tissue. These bags are often inside of other bags, or even inside of several bags. The largest bags define areas that we call body cavities. There are three main cavities: the thoracic cavity, the abdominal cavity and the pelvic cavity. The thoracic cavity is subdivided into three smaller cavities: the pleural cavity (containing the lungs), the mediastinum(in the middle), and the pericardial cavity (containing the heart). The pleural cavity is easy to understand because it simply contains the lungs. The pericardial cavity contains not only the heart itself, but the large blood vessels that come out of it, such as the aorta. The pericardial cavity is inside of the third cavity, the mediastinum. ("Media" means "middle" and "stinum" can refer to the "sternum," which is the bone that runs down the center of the ribcage.) The mediastinum contains not only the pericardial cavity but also part of the esophagus and trachea, the thymus (remember this organ from module 2 on the immune system?), and quite a few nerves and lymph nodes. The thin layers of connective tissues that surround these cavities are made primarily of collagen and elastin (produced by fibroblast cells) but they also contain some very tiny nerves and blood vessels, as well as cells that make serous fluid. As we've seen in the past few lessons, the diaphragm separates the thoracic cavity from the abdominal cavity. The abdominal cavity contains the stomach, the spleen, the tail of the pancreas, the last half of the duodenum, the small intestines, most of the large intestines, and the mesentery (thin layers of connective tissue that anchor the intestines to the back wall of the abdominal cavity). -

Ligaments -Two-Layered Folds of Peritoneum That Attached the Lesser Mobile Solid Viscera to the Abdominal Wall
Ingegneria delle tecnologie per la salute Fondamenti di anatomia e istologia aa. 2019-20 Lesson 7. Digestive system and peritoneum Peritoneum, abdominal vessel and spleen PERITONEUM: General features = a thin serous membrane that line walls of abdominal and pelvic cavities and cover organs within these cavities •Parietal peritoneum -lines walls of abdominal and pelvic cavities •Visceral peritoneum -covers organs •Peritoneal cavity - potential space between parietal and visceral layer of peritoneum, in male, is a closed sac, but in female, there is a communication with exterior through uterine tubes, uterus, and vagina Function • Secretes a lubricating serous fluid that continuously moistens associated organs • Absorb • Support viscera Peritoneum Histology The peritoneum is a serosal membrane that consists of a single layer of mesothelial cells and is supported by a basement membrane. The layer is attached to the body wall and viscera by a glycosaminoglycan matrix that contains collagen fibers, vessels, nerves, macrophages, and fat cells. relationship between viscera and peritoneum • Intraperitoneal viscera -viscera completely surrounded by peritoneum, example, stomach, superior part of duodenum, jejunum, ileum, cecum, vermiform appendix, transverse and sigmoid colons, spleen and ovary • Interperitoneal viscera -most part of viscera surrounded by peritoneum, example, liver, gallbladder, ascending and descending colon, upper part of rectum, urinary bladder and uterus • Retroperitoneal viscera -some organs lie on the posterior abdominal -
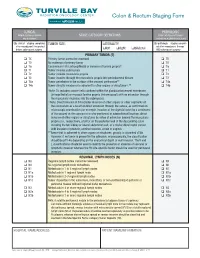
Colon & Rectum Staging Form
Colon & Rectum Staging Form CLINICAL PATHOLOGIC Extent of disease before STAGE CATEGORY DEFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical – staging completed TUMOR SIZE: LATERALITY: y pathologic – staging complet- after neoadjuvant therapy but ed after neoadjuvant therapy before subsequent surgery left right bilateral AND subsequent surgery PRIMARY TUMOR (T) TX Primary tumor cannot be assessed TX T0 No evidence of primary tumor T0 Tis Carcinoma in situ: intraepithelial or invasion of lamina propria* Tis T1 Tumor invades submucosa T1 T2 Tumor invades muscularis propria T2 T3 Tumor invades through the muscularis propria into pericolorectal tissues T3 T4a Tumor penetrates to the surface of the visceral peritoneum** T4a T4b Tumor directly invades or is adherent to other organs or structures^,** T4b *Note: Tis includes cancer cells confined within the glandular basement membrane (intraepithelial) or mucosal lamina propria (intramucosal) with no extension through the muscularis mucosae into the submucosa. ^Note: Direct invasion in T4 includes invasion of other organs or other segments of the colorectum as a result of direct extension through the serosa, as confirmed on microscopic examination (for example, invasion of the sigmoid colon by a carcinoma of the cecum) or, for cancers in a retro-peritoneal or subperitoneal location, direct invasion of other organs or structures by virtue of extension beyond the muscularis propria (i.e., respectively, a tumor on the posterior wall of the descending colon invading the left kidney or lateral abdominal wall; or a mid or distal rectal cancer with invasion of prostate, seminal vesicles, cervix or vagina). **Tumor that is adherent to other organs or structures, grossly, is classified cT4b. -

The Formation of Peritoneal Adhesions
THE FORMATION OF PERITONEAL ADHESIONS Christian DellaCorte, Ph.D., C.M.T. The increased incidence of postoperative adhesions and their complications has focused attention on trying to understand the adhesion, adhesion formation, clinical consequences, and prevention of adhesion formation. Adhesions are highly differentiated, formed through an intricate process involving a complex organ, the peritoneum, whose surface lining is the key site in adhesion formation. The peritoneum, a serous membrane, serves a protective function for the contents of the abdominal cavity. Homeostasis is maintained by allowing exchange of molecules and production of peritoneal fluid. This provides an environment for optimal function of intra-abdominal organs. Forms of trauma to the peritoneum (i.e., mechanical, thermal, chemical, infectious, surgical, and/or ischemic) can result in the formation of peritoneal adhesions. In 1919, it was shown that peritoneal healing differed from that of skin. When the peritoneal membrane is traumatized, a dynamic response results that produces a series of steps toward rapid regeneration in approximately five to seven days of the injured peritoneum via re-epithelialization, irrespective of the size of injury. Microscopic studies showed the new peritoneal cells are derived from mesodermal cells of the underlying granulation tissue, multipotent mesenchymal cells that are able to take the form of fibroblasts or mesothelial cells. When a defect is made in the parietal peritoneum the entire surface becomes simultaneously epithelialized, differing from the gradual epidermalization from the borders as is found in skin wounds. Multiplication and migration of mesothelial cells from the margins of the wound may play a small part in the regenerative process, but it does not play a major role. -

Magnetic Resonance Enterography Findings of a Gastrocolic Fistula in Crohn’S Disease
Letter to the Editor Magnetic resonance enterography findings of a gastrocolic fistula in Crohn’s disease Sanne N. van Munster1, Mark F. J. Stolk2, Karel C. Kuypers3, Rene Wiezer1, Thomas L. Bollen4 1Department of Surgery, 2Department of Gastroenterology and Hepatology, 3Department of Pathology, 4Department of Radiology, Sint Antonius Ziekenhuis, Nieuwegein, The Netherlands Correspondence to: Drs. Sanne N. van Munster. AMC, Academisch Medisch Centrum Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands. Email: [email protected]. Submitted Jul 09, 2016. Accepted for publication Jul 30, 2016. doi: 10.21037/qims.2016.08.06 View this article at: http://dx.doi.org/10.21037/qims.2016.08.06 Crohn’s disease (CD) is characterized by patches of Definitive treatment was established by segment inflammation, which may affect the whole gastro-intestinal resection of the splenic flexure with stapling of the tract. Internal fistulization is a common complication of CD gastrocolic fistula Figure( 1). The postoperative course was due to the transmural nature of inflammation. However, unremarkable and patient recovered uneventfully. gastrocolic fistulas are rare in CD. We present the magnetic Gross pathological examination of the surgical specimen resonance enterography (MRE) findings of a gastrocolic confirmed the presence of fistulous disease. A deep fistula in a patient with longstanding CD with clinical and penetrating inflammatory process originated from the pathologic correlation. colonic mucosa, extended through the colonic wall and attached to the stomach. In this inflammatory tract, gastric mucosa was found represented by glands formed by parietal Case presentation and chief cells of fundic mucosa. A 29-year-old woman with perianal fistulizing CD visited our hospital for left-sided upper abdominal pain starting Discussion about 30 minutes after the meals, bloating, diarrhea, anorexia, and weight loss. -

Porta Hepatis) - Bile Ducts, Portal Vein, Hepatic Arteries
10 Al-Mohtaseb Faisal Nimri Shada gharaibeh The Liver continued The superior surface of the liver You can see * The right and left lobes. * Cut edge of the Falciform ligament. * The coronary ligament, continues on both sides as: * The left triangular ligament * The right triangular ligament * Between the edges of the coronary ligament is the Bare area of the liver (where there is no peritoneum covering the liver). * Groove for the inferior vena cava and the 3 hepatic veins that drain in it. * Cut edge of the Falciform ligament. * Caudate lobe of the liver more or less wrapping around the groove of the inferior vena cava * Fundus of gall bladder * Ligamentum teres → Relations of the superior surface • Diaphragm (the diaphragm is above the liver and is related to the anterior, superior and posterior surfaces of the liver but the visceral surface of the liver doesn’t have relations with the diaphragm). The diaphragm separates the Pleura & lung and the Pericardium & heart from the liver. 1 | P a g e → Relations of the liver anteriorly • Diaphragm • Rt & Lt pleura and lung (separated from the liver by the diaphragm) • Costal cartilage • Xiphoid process • Anterior abdominal wall → Relations of the liver posteriorly • Diaphragm • Rt. Kidney • Supra renal gland • Transverse colon (hepatic flexure) • Duodenum • Gall bladder • I.V.C • Esophagus • Fundus of stomach (pay attention to the impressions in the picture they are important) → lobes of the liver • Right Lobe • Left lobe • Quadrate lobe • Caudate lobe (the quadrate and caudate lobes are similar