MIT 9.14 Class 9B-10A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cranial Nerves
Cranial Nerves (1,5,7,8,9,10,11 and 12) Slides not included 9th and 10th Cranial 11th and 12th Cranial 8th Cranial Nerve 5th and 7th Cranial 1st Cranial Nerve Nerves Nerves Nerves (3,7,11,12,13,21,23,24) - (10,16) (12,23) Slides included: (14 to 17) *Slides that are not included mostly are slides of summaries or pictures. Nouf Alabdulkarim. Med 435 Olfactory Nerve [The 1st Cranial Nerve] Special Sensory Olfactory pathway 1st order neuron Receptors Axons of 1st order Neurons Olfactory receptors are specialized, ciliated nerve cells The axons of these bipolar cells 12 -20 fibers form the that lie in the olfactory epithelium. true olfactory nerve fibers. Which passes through the cribriform plate of ethmoid → They join the olfactory bulb Preliminary processing of olfactory information It is within the olfactory bulb, which contains interneurones and large Mitral cells; axons from the latter leave the bulb to form the olfactory tract. nd 2 order neuron • It is formed by the Mitral cells of olfactory bulb. • The axons of these cells form the olfactory tract. • Each tract divides into 2 roots at the anterior perforated substance: Lateral root Medial root Carries olfactory fibers to end in cortex of the Uncus & • crosses midline through anterior commissure adjacent part of Hippocampal gyrus (center of smell). and joins the uncrossed lateral root of opposite side. • It connects olfactory centers of 2 cerebral hemispheres. • So each olfactory center receives smell sensation from both halves of nasal cavity. NB. Olfactory pathway is the only sensory pathway which reaches the cerebral cortex without passing through the Thalamus . -

The Use of Cholinesterase Techniques to Study Topographical Localization in the Hypoglossal Nucleus of the Rat
J. Anat. (1971), 110, 2, pp. 203-213 203 With 1O figures Printed in Great Britain The use of cholinesterase techniques to study topographical localization in the hypoglossal nucleus of the rat P. R. LEWIS*, B. A. FLUMERFELTt AND C. C. D. SHUTE* Department ofAnatomy, University of Cambridge (Accepted 4 August 1971) INTRODUCTION The hypoglossal nucleus is perhaps the most suitable motor nucleus for the experi- mental study of the cytological changes occurring in cholinergic neurons following axotomy. The cells are large and the nucleus is easy to find even in a fresh unfixed brain; furthermore, the nucleus is so close to the midline that it is possible to use one side as a control for the other with complete confidence and to view equivalent control and experimental neurons simultaneously at quite high magnifications. An added advantage is that the hypoglossal nerve trunk in the neck region is almost purely motor; the central effects of axotomy are therefore not complicated by any significant loss of sensory fibres. Our interest in the nucleus was heightened by the discovery that in the rat a group of neurons at the caudal end contained a high concentration of an enzyme resembling in its histochemical reactions pseudocholin- esterase (Shute & Lewis, 1963). The enzyme will hydrolyse acetylthiocholine and is inhibited by ethopropazine, but its most characteristic property is a rapid hydrolysis of butyrylthiocholine; BuChE would thus seem an appropriate abbreviation to distinguish it from true cholinesterase (AChE), the enzyme typically present in motor neurons. It was shown originally by Schwarzacher (1958) that there is a marked decrease in AChE activity in hypoglossal neurons during the second and third weeks following axotomy (although he also looked at the response of pseudocholinesterase he did not comment on the specifically staining group of cells). -

Cellular Changes in Injured Rat Spinal Cord Following Electrical Brainstem Stimulation
brain sciences Article Cellular Changes in Injured Rat Spinal Cord Following Electrical Brainstem Stimulation Walter J. Jermakowicz 1,* , Stephanie S. Sloley 2, Lia Dan 2, Alberto Vitores 2, Melissa M. Carballosa-Gautam 2 and Ian D. Hentall 2 1 Department of Neurological Surgery, University of Miami, 1095 NW 14th Terr, Miami, FL 33136, USA 2 Miami Project to Cure Paralysis, University of Miami, 1095 NW 14th Terr., Miami, FL 33136, USA; [email protected] (S.S.S.); [email protected] (L.D.); [email protected] (A.V.); [email protected] (M.M.C.-G.); [email protected] (I.D.H.) * Correspondence: [email protected]; Tel.: +1-615-818-3070 Received: 6 May 2019; Accepted: 27 May 2019; Published: 28 May 2019 Abstract: Spinal cord injury (SCI) is a major cause of disability and pain, but little progress has been made in its clinical management. Low-frequency electrical stimulation (LFS) of various anti-nociceptive targets improves outcomes after SCI, including motor recovery and mechanical allodynia. However, the mechanisms of these beneficial effects are incompletely delineated and probably multiple. Our aim was to explore near-term effects of LFS in the hindbrain’s nucleus raphe magnus (NRM) on cellular proliferation in a rat SCI model. Starting 24 h after incomplete contusional SCI at C5, intermittent LFS at 8 Hz was delivered wirelessly to NRM. Controls were given inactive stimulators. At 48 h, 5-bromodeoxyuridine (BrdU) was administered and, at 72 h, spinal cords were extracted and immunostained for various immune and neuroglial progenitor markers and BrdU at the level of the lesion and proximally and distally. -

Hemisus Marmoratum
Neuroscience Letters 244 (1998) 5–8 Distribution of hypoglossal motor neurons innervating the prehensile tongue of the African pig-nosed frog, Hemisus marmoratum Curtis W. Andersona,*, Kiisa C. Nishikawab, Joyce Keifera aDepartment of Anatomy and Structural Biology, University of South Dakota School of Medicine, Vermillion, SD 57069, USA bDepartment of Biological Sciences, Physiology and Functional Morphology Group, Northern Arizona University, Flagstaff, AZ 86011, USA Received 15 December 1997; received in revised form 28 January 1998; accepted 28 January 1998 Abstract Using retrograde neuronal tracers, a study of the distribution of hypoglossal motor neurons innervating the tongue musculature was performed in the African pig-nosed frog, Hemisus marmoratum. This species is a radically divergent anuran amphibian with a prehensile tongue that can be aimed in three dimensions relative to the head. The results illustrate a unique rostrocaudal distribution of the ventrolateral hypoglossal nucleus and an unusually large number of motor neurons within this cell group. During the evolution of the long, prehensile tongue of Hemisus, the motor neurons innervating the tongue have greatly increased in number and have become more caudally distributed in the brainstem and spinal cord compared to other anurans. These observations have implications for understanding neuronal reconfiguring of motoneurons for novel morphologies requiring new muscle activation patterns. 1998 Elsevier Science Ireland Ltd. Keywords: Anuran amphibian; Hypoglossal nerve; Motor nuclei; Tongue Recent studies of feeding in anurans have revealed a hylidae [12,15]. In hydrostatic elongation, the tongue diversity of tongue morphologies and feeding mechanisms protractor muscle (M. genioglossus) has two types of mus- [1,5,7,8,10,11,15]. -

Neuromodulation Shapes Interneuron Communication in the Mouse Striatum
From DEPARTMENT OF NEUROSCIENCE Karolinska Institutet, Stockholm, Sweden NEUROMODULATION SHAPES INTERNEURON COMMUNICATION IN THE MOUSE STRIATUM Matthijs Constantijn Dorst Stockholm 2020 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by US-AB © Matthijs Constantijn Dorst, 2020 ISBN 978-91-7831-908-4 Neuromodulation shapes interneuron communication in the mouse Striatum THESIS FOR DOCTORAL DEGREE (Ph.D.) By Matthijs Constantijn Dorst Principal Supervisor: Opponent: Professor Gilad Silberberg Professor Hagai Bergman Karolinska Institutet The Hebrew University of Jerusalem Department of Neuroscience Edmond & Lily Safra Center for Brain Sciences Co-supervisor(s): Examination Board: Professor Per Uhlén Professor Per Svenningsson Karolinska Institutet Karolinska Institutet Department of Medical Biochemistry and Department of Clinical Neuroscience Biophysics Division of Neuropharmacology - movement disorders Senior lecturer Karima Chergui Karolinska Institutet Department of Physiology and Pharmacology Division of Molecular Neurophysiology Professor Klas Kullander Uppsala Universitet Department of Neuroscience Research group Formation and Function of Neuronal Circuits Included Studies The following studies are included in this thesis, and will be referenced through- out the text as such: Study 1 Garas, F.N., Shah, R.S., Kormann, E., Doig, N.M., Vinciati, F., Nakamura, K.C., Dorst, M.C., Smith, Y., Magill, P.J. and Sharott, A., 2016. Sec- retagogin expression delineates functionally-specialized populations of striatal parvalbumin-containing interneurons. Elife, 5, p.e16088. Study 2 Lindroos, R., Dorst, M.C., Du, K., Filipović, M., Keller, D., Ketzef, M., Kozlov, A.K., Kumar, A., Lindahl, M., Nair, A.G., Pérez-Fernández, J., Grillner, S., Silberberg, G., Kotaleski, J.H., 2018. Basal Ganglia Neuromodulation Over Multiple Temporal and Structural Scales—Simulations of Direct Pathway MSNs Investigate the Fast Onset of Dopaminergic Effects and Predict the Role of Kv4. -

Role of Glucocorticoids in Tuning Hindbrain Stress Integration
The Journal of Neuroscience, November 3, 2010 • 30(44):14907–14914 • 14907 Cellular/Molecular Role of Glucocorticoids in Tuning Hindbrain Stress Integration Rong Zhang ( ),1,3 Ryan Jankord,1 Jonathan N. Flak,1 Matia B. Solomon,1 David A. D’Alessio,1,2 and James P. Herman1 Departments of 1Psychiatry and 2Internal Medicine, University of Cincinnati, Cincinnati, Ohio 45237, and 3Division of Endocrinology, Children’s Hospital Boston, Harvard Medical School, Boston, Massachusetts 02115 The nucleus of the solitary tract (NTS) is a critical integrative site for coordination of autonomic and endocrine stress responses. Stress-excitatory signals from the NTS are communicated by both catecholaminergic [norepinephrine (NE), epinephrine (E)] and non- catecholaminergic [e.g., glucagon-like peptide-1 (GLP-1)] neurons. Recent studies suggest that outputs of the NE/E and GLP-1 neurons of the NTS are selectively engaged during acute stress. This study was designed to test mechanisms of chronic stress integration in the paraventricular nucleus, focusing on the role of glucocorticoids. Our data indicate that chronic variable stress (CVS) causes downregu- lation of preproglucagon (GLP-1 precursor) mRNA in the NTS and reduction of GLP-1 innervation to the paraventricular nucleus of the hypothalamus. Glucocorticoids were necessary for preproglucagon (PPG) reduction in CVS animals and were sufficient to lower PPG mRNA in otherwise unstressed animals. The data are consistent with a glucocorticoid-mediated withdrawal of GLP-1 in key stress circuits. In contrast, expression of tyrosine hydroxylase mRNA, the rate-limiting enzyme in catecholamine synthesis, was increased by stress in a glucocorticoid-independent manner. These suggest differential roles of ascending catecholamine and GLP-1 systems in chronic stress, with withdrawal of GLP-1 involved in stress adaptation and enhanced NE/E capacity responsible for facilitation of responses to novel stress experiences. -

Nonthermal and Reversible Control of Neuronal Signaling and Behavior by Midinfrared Stimulation
Nonthermal and reversible control of neuronal signaling and behavior by midinfrared stimulation Xi Liua,b,1, Zhi Qiaoc,d,1, Yuming Chaie,f,1, Zhi Zhug,1, Kaijie Wuc, Wenliang Jih, Daguang Lie,f, Yujie Xiaoa,b, Lanqun Maoh, Chao Changc,d,2, Quan Wene,f,2, Bo Songg,2, and Yousheng Shui,2 aState Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, Beijing 100875, China; bIDG/McGovern Institute for Brain Research, Beijing Normal University, Beijing 100875, China; cKey Laboratory for Physical Electronics and Devices of the Ministry of Education, School of Electronic and Information Engineering, Xi’an Jiaotong University, Xi’an, Shaanxi 710049, China; dInnovation Laboratory of Terahertz Biophysics, National Innovation Institute of Defense Technology, Beijing 100071, China; eHefei National Laboratory for Physical Sciences at the Microscale Center for Integrative Imaging, School of Life Sciences, University of Science and Technology of China, Hefei 230027, China; fKey Laboratory of Brain Function and Disease, Chinese Academy of Sciences, Hefei 230027, China; gSchool of Optical-Electrical Computer Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China; hBeijing National Laboratory for Molecular Sciences, Key Laboratory of Analytical Chemistry for Living Biosystems, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China; and iDepartment of Neurology, Huashan Hospital, State Key Laboratory of Medical Neurobiology, Institute for Translational Brain Research, MOE Frontiers Center for Brain Science, Fudan University, Shanghai 200032, China Edited by Lily Yeh Jan, University of California, San Francisco, CA, and approved January 17, 2021 (received for review August 5, 2020) Various neuromodulation approaches have been employed to spiking activity in rodent somatosensory cortex (7) and nonhu- alter neuronal spiking activity and thus regulate brain functions man primate visual cortex in vivo (8). -

Probing Forebrain to Hindbrain Circuit Functions in Xenopus
Received: 15 November 2016 | Accepted: 16 November 2016 DOI 10.1002/dvg.22999 REVIEW Probing forebrain to hindbrain circuit functions in Xenopus Darcy B. Kelley1 | Taffeta M. Elliott2 | Ben J. Evans3 | Ian C. Hall4 | Elizabeth C. Leininger5 | Heather J. Rhodes6 | Ayako Yamaguchi7 | Erik Zornik8 1Department of Biological Sciences, Columbia University, New York, New York Abstract 10027 The vertebrate hindbrain includes neural circuits that govern essential functions including breath- 2Department of Psychology, New Mexico ing, blood pressure and heart rate. Hindbrain circuits also participate in generating rhythmic motor Tech, Socorro, New Mexico 87801 patterns for vocalization. In most tetrapods, sound production is powered by expiration and the 3 Department of Biology, McMaster circuitry underlying vocalization and respiration must be linked. Perception and arousal are also University, Hamilton, Ontario, Ontario linked; acoustic features of social communication sounds—for example, a baby’scry—can drive L8S4K1, Canada autonomic responses. The close links between autonomic functions that are essential for life and 4Department of Biology, Benedictine University, Lisle, Illinois vocal expression have been a major in vivo experimental challenge. Xenopus provides an opportu- 5Department of Biology, St. Mary’s College, nity to address this challenge using an ex vivo preparation: an isolated brain that generates vocal St. Mary’s City, Maryland 29686 and breathing patterns. The isolated brain allows identification and manipulation of hindbrain vocal 6Department of Biology, Denison University, circuits as well as their activation by forebrain circuits that receive sensory input, initiate motor Granville, Ohio 43023 patterns and control arousal. Advances in imaging technologies, coupled to the production of Xen- 7 Department of Biology, University of Utah, opus lines expressing genetically encoded calcium sensors, provide powerful tools for imaging Salt Lake City, Utah 84112 neuronal patterns in the entire fictively behaving brain, a goal of the BRAIN Initiative. -

Neuroanatomy
Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Neuroanatomy W. Jeffrey Wilson Fall 2012 \Without education we are in a horrible and deadly danger of taking educated people seriously." { Gilbert Keith Chesterton [LATEX in use { a Microsoft- & PowerPoint-free presentation] Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Blood-Brain Barrier Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Peripheral Nervous System • Somatic N.S.: skeletal muscles, skin, joints • Autonomic N.S.: internal organs, glands • Sympathetic N.S.: rapid expenditure of energy • Parasympathetic N.S.: restoration of energy Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Spinal Cord Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain | Ventricles Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Hindbrain Myelencephalon & Metencephalon Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Reticular -

Is Essential for the Development, Survival and Function of Hypoglossal
© 2019. Published by The Company of Biologists Ltd | Development (2019) 146, dev174045. doi:10.1242/dev.174045 RESEARCH ARTICLE Teashirt 1 (Tshz1) is essential for the development, survival and function of hypoglossal and phrenic motor neurons in mouse Charlotte Chaimowicz1, Pierre-Louis Ruffault1, Cyril Chéret1,*, Andrew Woehler2, NiccolòZampieri3, Gilles Fortin4, Alistair N. Garratt5 and Carmen Birchmeier1,‡ ABSTRACT the phrenic motor column located in the mid-cervical spinal cord. Feeding and breathing are essential motor functions and rely on the The tongue has crucial functions in feeding and is innervated by activity of hypoglossal and phrenic motor neurons that innervate the the hypoglossal motor neurons in the lower brainstem. The activity tongue and diaphragm, respectively. Little is known about the genetic of phrenic and hypoglossal motor neurons has to be tightly programs that control the development of these neuronal subtypes. coordinated to avoid maladaptive outcomes such as the swallowing The transcription factor Tshz1 is strongly and persistently expressed of air or the blockage of airways (Moore et al., 2014). Hence, in developing hypoglossal and phrenic motor neurons. We used hypoglossal and phrenic motor neurons and the circuitry that conditional mutation of Tshz1 in the progenitor zone of motor neurons coordinates their activity are essential for animal survival. (Tshz1MNΔ) to show that Tshz1 is essential for survival and function of Nevertheless, relatively little is known about the formation of the hypoglossal and phrenic motor neurons. Hypoglossal and phrenic motor neurons that relay breathing and feeding commands. motor neurons are born in correct numbers, but many die between All motor neurons that innervate skeletal muscle, i.e. -
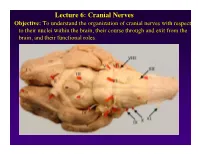
Lecture 6: Cranial Nerves
Lecture 6: Cranial Nerves Objective: To understand the organization of cranial nerves with respect to their nuclei within the brain, their course through and exit from the brain, and their functional roles. Olfactory Eye Muscles 3, 4 &6 Cranial Nerves 1-7 I overview Table, Page 49 II Lecture notes Cranial Nerves and their Functions V Trigeminal VII Facial VIII IX X XII XI Cranial Nerves 8-12 Overview sternocephalic I. Factors Responsible for the Complex Internal Organization of the Brain Stem-> leads to altered location of cranial nerve nuclei in adult brain stem 1. Development of the Fourth Ventricle a. Medulla and Pons develop ventral to the 4th ventricle cerebellum b. Alar plate is displaced lateral to basal plate 4 Medulla Developing Neural Tube 2. Cranial nerve nuclei form discontinuous columns Rostral 12 SE Page 48 Notes 3. Some cranial nerve nuclei migrate from their primitive embryonic positions (e.g., nuclei of V and VII) Facial N. Factors responsible for the complex internal organization of the brainstem: 4) Special senses develop in association with the brain stem. Nuclei of special senses 5) Development of the cerebellum and its connections Cerebellum II. Cranial Nerve Nuclei: Nucleus = column of neuron cell bodies. Efferent nuclei are composed of cell bodies of alpha or gamma motor neurons (SE) or preganglionic parasympathetic neurons (VE). III. Motor Efferent Nuclei (Basal Plate Derivatives): 1. SE (Somatic Efferent) Nuclei: SE neurons form two longitudinally oriented but discontinuous columns of cell bodies in the brain stem. Neurons that comprise these columns are responsible for innervating all of the skeletal musculature of the head. -

Appearance and Transient Expression of Oxytocin Receptors in Fetal, Infant, and Peripubertal -Rat Brain Studied by Autoradiography and Electrophysiology
The Journal of Neuroscience, May 1969, g(5): 1764-I 773 Appearance and Transient Expression of Oxytocin Receptors in Fetal, Infant, and Peripubertal -Rat Brain Studied by Autoradiography and Electrophysiology Eliane Tribollet,’ Serge Charpak,’ Anne Schmidt,* Michel Dubois-Dauphin,’ and Jean Jacques Dreifussl ‘Department of Physiology, University Medical Center, Geneva, Switzerland, and ‘CNRS-INSERM, Center of Pharmacology-Endocrinology, Montpellier, France The development of oxytocin (OT) receptors in the rat brain The classicalhormonal effects of oxytocin (OT) releasedfrom and spinal cord was studied by in vitro light microscopic the hypothalamoneurohypophyseal axons are well established. autoradiography and by electrophysiology. OT receptors were In addition, OT is also present in axons projecting to various labeled using a monoiodinated OT antagonist in tissue sec- areaswithin the CNS (Sofroniew, 1985), which suggeststhat it tions from animals aged between embryonic day 12 (E12) may play a neurotransmitter or neuromodulator role. Addi- and postnatal day 90 (PN90); the response of ongoing spike tional evidence for such a role includes its releaseunder exper- activity to the addition of OT was assessed in neurons lo- imental conditions in situ and in vitro (Buijs, 1983), its effect cated in the dorsal motor nucleus of the vagus nerve of the on the electrical activity of single neurons in the brain (Mtihle- neonate. thaler et al., 1983, 1984; Charpak et al., 1984), the fact that Specific binding was detected first at El4 in a region that small amounts of OT injected into the brain or the cerebral later differentiated into the dorsal motor nucleus of the vagus ventricles modulate neuroendocrine and autonomic functions nerve.