Public Health Pesticides: an Inventory of Chemical Tools for Vector Control
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Detecting Traces of Methyl Eugenol in Essential Oils
Detecting traces of methyl eugenol in essential oils Ian Southwella,Mike Russellband Noel Daviesc Introduction Regulatory authorities are becoming more concerned about even low levels of suspected toxins in fl avour, fragrance and medicinal applications1. For example, allyl alkoxybenzenes (eg safrole, estragole (methyl chavicol), methyl eugenol), common constituents of many essential oils2,3, are of concern because high doses have caused cancer in rodents4-6. This concern is often exaggerated considering that: ► safety threshold doses some 1000 times less than the offending concentration recommended (eg IFRA Standards5) ► carcinogenesis is not always considered a threshold phenomenon7-9 ► the validity of dose extrapolation from rodents to humans is uncertain10-11 ► many complete essential oils also contain anti-carcinogenic congeners12-14 Hence reliable methods for the determination of trace quantities of allyl alkoxybenzenes in essential oils and formulated products are essential. Determinations Gas Chromatography using FID and MS detection Because of the absence of competing mass spectral ions illustrated the complexity of these essential oils and the from Melaleuca oil components, GCMS in SIM mode using diffi culties encountered in locating trace constituents. the m/z 178 ion (Varian Factor-Four VF-5ms, 30m x 0.25mm id, 0.25 μm fi lm) facilitated the determination of methyl Using tea tree oil, “Oil of Melaleuca, terpinen-4-ol type” as eugenol (Figure 2). a model, careful column selection for GC-FID (DB 1701 Length 60 m x 0.25 mm Dia x 0.25 μm fi lm (mid polarity) ) Such SIM traces, although excellent for determining analytes facilitated the separation and determination of methyl like methyl eugenol, give a false impression of the complexity eugenol to the ppm level (Figure 1). -

Manual for Certificate Course on Plant Protection & Pesticide Management
Manual for Certificate Course on Plant Protection & Pesticide Management (for Pesticide Dealers) For Internal circulation only & has no legal validity Compiled by NIPHM Faculty Department of Agriculture , Cooperation& Farmers Welfare Ministry of Agriculture and Farmers Welfare Government of India National Institute of Plant Health Management Hyderabad-500030 TABLE OF CONTENTS Theory Practical CHAPTER Page No. class hours hours I. General Overview and Classification of Pesticides. 1. Introduction to classification based on use, 1 1 2 toxicity, chemistry 2. Insecticides 5 1 0 3. fungicides 9 1 0 4. Herbicides & Plant growth regulators 11 1 0 5. Other Pesticides (Acaricides, Nematicides & 16 1 0 rodenticides) II. Pesticide Act, Rules and Regulations 1. Introduction to Insecticide Act, 1968 and 19 1 0 Insecticide rules, 1971 2. Registration and Licensing of pesticides 23 1 0 3. Insecticide Inspector 26 2 0 4. Insecticide Analyst 30 1 4 5. Importance of packaging and labelling 35 1 0 6. Role and Responsibilities of Pesticide Dealer 37 1 0 under IA,1968 III. Pesticide Application A. Pesticide Formulation 1. Types of pesticide Formulations 39 3 8 2. Approved uses and Compatibility of pesticides 47 1 0 B. Usage Recommendation 1. Major pest and diseases of crops: identification 50 3 3 2. Principles and Strategies of Integrated Pest 80 2 1 Management & The Concept of Economic Threshold Level 3. Biological control and its Importance in Pest 93 1 2 Management C. Pesticide Application 1. Principles of Pesticide Application 117 1 0 2. Types of Sprayers and Dusters 121 1 4 3. Spray Nozzles and Their Classification 130 1 0 4. -

Complaint for Declaratory and Injunctive Relief 1 1 2 3 4 5 6 7 8 9
1 Justin Augustine (CA Bar No. 235561) Jaclyn Lopez (CA Bar No. 258589) 2 Center for Biological Diversity 351 California Street, Suite 600 3 San Francisco, CA 94104 Tel: (415) 436-9682 4 Fax: (415) 436-9683 [email protected] 5 [email protected] 6 Collette L. Adkins Giese (MN Bar No. 035059X)* Center for Biological Diversity 8640 Coral Sea Street Northeast 7 Minneapolis, MN 55449-5600 Tel: (651) 955-3821 8 Fax: (415) 436-9683 [email protected] 9 Michael W. Graf (CA Bar No. 136172) 10 Law Offices 227 Behrens Street 11 El Cerrito, CA 94530 Tel: (510) 525-7222 12 Fax: (510) 525-1208 [email protected] 13 Attorneys for Plaintiffs Center for Biological Diversity and 14 Pesticide Action Network North America *Seeking admission pro hac vice 15 16 IN THE UNITED STATES DISTRICT COURT 17 FOR THE NORTHERN DISTRICT OF CALIFORNIA 18 SAN FRANCISCO DIVISION 19 20 CENTER FOR BIOLOGICAL ) 21 DIVERSITY, a non-profit organization; and ) Case No.__________________ PESTICIDE ACTION NETWORK ) 22 NORTH AMERICA, a non-profit ) organization; ) 23 ) Plaintiffs, ) COMPLAINT FOR DECLARATORY 24 ) AND INJUNCTIVE RELIEF v. ) 25 ) ENVIRONMENTAL PROTECTION ) 26 AGENCY; and LISA JACKSON, ) Administrator, U.S. EPA; ) 27 ) Defendants. ) 28 _____________________________________ ) Complaint for Declaratory and Injunctive Relief 1 1 INTRODUCTION 2 1. This action challenges the failure of Defendants Environmental Protection Agency and 3 Lisa Jackson, Environmental Protection Agency Administrator, (collectively “EPA”) to consult with the 4 United States Fish and Wildlife Service (“FWS”) and National Marine Fisheries Service (“NMFS”) 5 (collectively “Service”) pursuant to Section 7(a)(2) of the Endangered Species Act (“ESA”), 16 U.S.C. -
Approved Uses of Registered Insecticides (Crop Based)
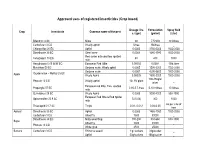
Approved uses of registered insecticides (Crop based) Dosage / ha Formulation Spray fluid Crop Insecticide Common name of the pest a.i (gm) (gm/ml) (Liter) Bifenthrin 8 SC Mites 60 7.5ml/lit 10 lit/tree Carbofuran 3 CG Woolly aphid 5/tree 166/tree _ Chlorpyrifos 20 EC Aphid 0.0005 3750-5000 1500-2000 Dimethoate 30 EC Stem borer 0.0003 1485-1980 1500-2000 Red spider mite and two spotted Fenazaquin 10 EC 40 400 1000 mite Hexythiazox 5.45 W/W EC European Red Mite 0.00002 0.0004 10ltr./tree Malathion 50 EC Sanjose scale, Wooly aphid 0.0005 1500-2000 1500-2000 Sanjose scale 0.0007 4200-5600 1500-2000 Oxydemeton – Methyl 25 EC Apple Wooly Aphid 0.00025 1500-2000 1500-2000 100-150gm/ Phorate 10 CG Woolly aphid 10- 15/ plant _ plant European red Mite, Two spotted Propargite 57 EC 2.85-5.7 /tree 5-10 ml/tree 10 lit/tree mite Quinalphos 25 EC Wooly Aphid 0.0005 3000-4000 500-1000 European Red Mite & Red Spider Spiromesifen 22.9 SC 72(0.03) 300 1000 mite As per size of Thiacloprid 21.7 SC Thrips 0.01- 0.012 0.04-0.05 tree Apricot Dimethoate 30 EC Aphid 0.0003 1485-1980 1500-2000 Carbofuran 3 CG Shoot fly 1500 50000 _ Dimethoate 30 EC Milky weed bug 180-200 594-660 500-1000 Bajra Shoot fly 3000 30000 _ Phorate 10 CG White grub 2500 25000 _ Banana Carbofuran 3 CG Rhizome weevil 1 g/ suckers 33g/sucker _ Aphid 50g/suckers 166g/sucker _ Nematode 1.5g/suckers 50g/suckers _ Dimethoate 30 EC Aphid, Lace wing bug 0.0003 1485-1980 1500-2000 Tingyi bug 0.00025 1500-2000 1500-2000 Oxydemeton – Methyl 25 EC Aphids 0.0005 3000-4000 1500-2000 2.5 -1.25/ 25 -12.5/ Phorate 10 CG Aphid _ plant plant Quinalphos 25 EC Tingid bug 0.0005 3000-4000 500-1000 Phosalone 35 EC Aphid 500 1428 500-1000 Aphid 1000 33300 _ Barely Carbofuran 3 CG Jassid 1250 41600 _ Cyst nematode 1000 33300 _ Phorate 10 CG Aphid 1000 10000 _ Beans Chlorpyrifos 20 EC Pod borer , Black bug 600 3000 500-1000 Chlorantraniliprole 18.5 SC Pod borers 25 125 500 Chlorpyrifos 1.5 DP Helicoverpa armigera 375 25000 _ Azadirachtin 0.03 (300 PPM) Pod Borer _ _ _ Bacillus thuringiensis Var. -

Cyantraniliprole
PUBLIC RELEASE SUMMARY on the Evaluation of the New Active Constituent Cyantraniliprole in the Product DuPont Exirel Insecticide APVMA Product Number 64103 OCTOBER 2013 © Australian Pesticides and Veterinary Medicines Authority 2013 ISSN: 1443-1335 (electronic) ISBN: 978-1-922188-50-2 (electronic) Ownership of intellectual property rights in this publication Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Australian Pesticides and Veterinary Medicines Authority (APVMA). Creative Commons licence With the exception of the Coat of Arms and other elements specifically identified, this publication is licensed under a Creative Commons Attribution 3.0 Australia Licence. This is a standard form agreement that allows you to copy, distribute, transmit and adapt this publication provided that you attribute the work. A summary of the licence terms is available from www.creativecommons.org/licenses/by/3.0/au/deed.en. The full licence terms are available from www.creativecommons.org/licenses/by/3.0/au/legalcode. The APVMA’s preference is that you attribute this publication (and any approved material sourced from it) using the following wording: Source: licensed from the Australian Pesticides and Veterinary Medicines Authority (APVMA) under a Creative Commons Attribution 3.0 Australia Licence. In referencing this document the Australian Pesticides and Veterinary Medicines Authority should be cited as author, publisher and copyright owner. Use of the Coat of Arms The terms under which the Coat of Arms can be used are set out on the Department of the Prime Minister and Cabinet website (see www.dpmc.gov.au/guidelines). -

Amyris and Siam-Wood Essential Oils: Insect Activity of Sesquiterpenes Gretchen E
Entomology Publications Entomology 12-20-2009 Amyris and Siam-wood Essential Oils: Insect Activity of Sesquiterpenes Gretchen E. Paluch Iowa State University Junwei Zhu United States Department of Agriculture Lyric Bartholomay Iowa State University, [email protected] Joel R. Coats Iowa State University, [email protected] Follow this and additional works at: http://lib.dr.iastate.edu/ent_pubs Part of the Entomology Commons, Other Pharmacology, Toxicology and Environmental Health Commons, Parasitic Diseases Commons, and the Plant Sciences Commons The ompc lete bibliographic information for this item can be found at http://lib.dr.iastate.edu/ ent_pubs/363. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Book Chapter is brought to you for free and open access by the Entomology at Iowa State University Digital Repository. It has been accepted for inclusion in Entomology Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Amyris and Siam-wood Essential Oils: Insect Activity of Sesquiterpenes Abstract Recent investigations on the sesquiterpene-rich Amyris (Amyris balsamifera L.) and Siam-wood (Fokienia hodginsii L.) essential oils revealed significant arthropod repellency and toxicity responses. Amyris essential oil and one of its major components, elemol, were evaluated in laboratory bioassays and identified as effective mosquito repellents, specifically characterized by high levels of contact and minimal spatial repellency. Mosquito responses to catnip (Nepeta cataria L.) essential oil are characterized with high spatial activity, but lack significant contact repellency. Sampling within the staticair bioassay chamber with solid-phase microextraction provided measurements of the relative concentration and distribution of volatiles. -

CYANTRANILIPROLE (263) the First Draft Was Prepared by Mr. David Lunn New Zealand Food Safety Authority, Wellington, New Zealand
Cyantraniliprole 361 CYANTRANILIPROLE (263) The first draft was prepared by Mr. David Lunn New Zealand Food Safety Authority, Wellington, New Zealand EXPLANATION Cyantraniliprole is a diamide insecticide with a mode of action (ryanodine receptor activation) similar to chlorantraniliprole and flubendiamide. It has root systemic activity with some translaminar movement and is effective against the larval stages of lepidopteran insects; and also on thrips, aphids, and some other chewing and sucking insects. Authorisations exist for the use of cyantraniliprole in Canada, Columbia, Malaysia, New Zealand, Vietnam and the CLISS countries in West Africa. Authorisations are also being progressed in Australia, Europe and USA under an OECD Joint Review exercise. Cyantraniliprole was scheduled by the Forty-fourth Session of the CCPR as a new compound for consideration by the 2013 JMPR. Residue and analytical aspects of cyantraniliprole were considered for the first time by the present meeting. The manufacturer submitted studies on metabolism, analytical methods, supervised field trials, processing, freezer storage stability, environmental fate in soil and rotational crop residues. In this evaluation, the values presented in the tables are as reported in the various studies, but in the accompanying text, they have generally been rounded to two significant digits. Abbreviations have also been used for the various cyantraniliprole metabolites mentioned in the study reports. These include: IN-F6L99 3-Bromo-N-methyl-1H-pyrazole-5-carboxamide IN-HGW87 -

Effects of Cyantraniliprole, a Novel Anthranilic Diamide Insecticide, Against Asian Citrus Psyllid Under Laboratory and Field Co
Submitted to: Correspondence to: Pest Management Science Lukasz L. Stelinski, Entomology and Nematology Department, Citrus Research & Education Center, University of Florida, 700 Experiment Station Road, Lake Alfred, FL 33850. Ph: 863 956 8851. Fax: 863 956 4631. Email: [email protected] Effects of cyantraniliprole, a novel anthranilic diamide insecticide, against Asian citrus psyllid under laboratory and field conditions Siddharth Tiwari and Lukasz L. Stelinski* Entomology and Nematology Department, Citrus Research and Education Center, University of Florida, Lake Alfred, FL 33850 *Corresponding author Accepted Article This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/ps.3468. © 2012 Society of Chemical Industry Abstract BACKGROUND:The Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae), is the most destructive pest of citrus in Florida. The development of insecticide resistance in several populations of D. citrihas been documented. There is an urgent need to develop and integrate novel tools for the successful management of D. citri and also to prevent the development of insecticide resistance. RESULTS:We investigated the effects of a relatively newer chemistry, cyantraniliprole, against D. citri. The contact toxicity of cyantraniliprole was 297 fold higher against D. citrithan its primary parasitoid,Tamarixia radiata(Hymenoptera: Eulophidae). D. citrisettled and fed less on cyantraniliprole-treated plants than controls at concentrations as low as 0.025 and 0.125 µg AI mL-1, respectively. D. citri egg production, first instar emergence and adult emergence were significantly reduced on plants treated with 0.25, 0.02 and 0.25 µg AI mL-1of cyantraniliprole, respectively, when compared with control plants. -

Post-Fire Recovery of Woody Plants in the New England Tableland Bioregion
Post-fire recovery of woody plants in the New England Tableland Bioregion Peter J. ClarkeA, Kirsten J. E. Knox, Monica L. Campbell and Lachlan M. Copeland Botany, School of Environmental and Rural Sciences, University of New England, Armidale, NSW 2351, AUSTRALIA. ACorresponding author; email: [email protected] Abstract: The resprouting response of plant species to fire is a key life history trait that has profound effects on post-fire population dynamics and community composition. This study documents the post-fire response (resprouting and maturation times) of woody species in six contrasting formations in the New England Tableland Bioregion of eastern Australia. Rainforest had the highest proportion of resprouting woody taxa and rocky outcrops had the lowest. Surprisingly, no significant difference in the median maturation length was found among habitats, but the communities varied in the range of maturation times. Within these communities, seedlings of species killed by fire, mature faster than seedlings of species that resprout. The slowest maturing species were those that have canopy held seed banks and were killed by fire, and these were used as indicator species to examine fire immaturity risk. Finally, we examine whether current fire management immaturity thresholds appear to be appropriate for these communities and find they need to be amended. Cunninghamia (2009) 11(2): 221–239 Introduction Maturation times of new recruits for those plants killed by fire is also a critical biological variable in the context of fire Fire is a pervasive ecological factor that influences the regimes because this time sets the lower limit for fire intervals evolution, distribution and abundance of woody plants that can cause local population decline or extirpation (Keith (Whelan 1995; Bond & van Wilgen 1996; Bradstock et al. -

Use Date Issued: September 2006 ______
United States Environmental Protection Agency Office of Prevention, Pesticides and Toxic Substances (7505P) _______________________________________________________ Pesticide Fact Sheet Name of Chemical: Metofluthrin Reason for Issuance: New Chemical Nonfood Use Date Issued: September 2006 _______________________________________________________ Description of Chemical IUPAC name: 2,3,5,6-tetrafluoro-4-(methoxymethyl)benzyl (EZ)- (1RS,3RS;1RS,3SR)-2,2-dimethyl-3-prop-1- enylcyclopropanecarboxylate CAS name: [2,3,5,6-tetrafluoro-4-(methoxymethyl)phenyl]methyl 2,2-dimethyl-3-(1-propenyl)cyclopropanecarboxylate Common Name: Metofluthrin Empirical Formula: C18H20F4O3 EPA Chemical Code: 109709 Chemical Abstracts Service (CAS) Number: 240494-70-6 Chemical Class: Pyrethroid ester Registration Status: New Chemical, nonfood use Pesticide Type: Insecticide repellent not applied to human skin U.S.Technical Registrant : Sumitomo Chemical Company, LTD. 1330 Dillon Hghts. Ave. Baltimore, MD 21228 Use Pattern and Formulations Currently there are two end use products being proposed for metofluthrin. DeckMate ™ Mosquito Repellent Strip is an impregnated paper strip (~3,528 cm2) containing 1.82 percent metofluthrin as the active ingredient. The product also contains Bitrex ™ to discourage oral exposure to children or animals. The product is for use on patios, campsites, decks, cabanas, and other outdoor areas. One strip is applied per 10 ft × 10 ft outdoor area. Indoors the application rate is two strips per 50 m3. There are approximately 200 mg of metofluthrin initially in the strip. The strips can provide up to one week of protection Metofluthrin evaporates readily and therefore requires no external heat. Norm 1- is a personal outdoor insect repellent product consisting of a holder containing a replaceable cartridge insert coated with up to 50 mg of metofluthrin. -

Methyl Eugenol: Its Occurrence, Distribution, and Role in Nature, Especially in Relation to Insect Behavior and Pollination
Journal of Insect Science: Vol. 12 | Article 56 Tan and Nishida Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination Keng Hong Tan1a* and Ritsuo Nishida2b 1Tan Hak Heng, 20, Jalan Tan Jit Seng, 11200 Penang, Malaysia 2Laboratory of Chemical Ecology, Graduate School of Agriculture, Kyoto University, Kyoto, 606-8502, Japan Abstract This review discusses the occurrence and distribution (within a plant) of methyl eugenol in different plant species (> 450) from 80 families spanning many plant orders, as well as various roles this chemical plays in nature, especially in the interactions between tephritid fruit flies and plants. Keywords: allomone, attractant, Bactrocera, chemical ecology, floral fragrance, insect pollinators, plant–insect interactions, plant semiochemicals, sex pheromone, synomone, tephritid fruit flies Abbreviations: ME, methyl eugenol; RK, raspberry ketone Correspondence: a [email protected], b [email protected], *Corresponding author Editor: Todd Shelly was editor of this paper. Received: 28 April 2011, Accepted: 27 August 2011 Copyright : This is an open access paper. We use the Creative Commons Attribution 3.0 license that permits unrestricted use, provided that the paper is properly attributed. ISSN: 1536-2442 | Vol. 12, Number 56 Cite this paper as: Tan KH, Nishida R. 2012. Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. Journal of Insect Science 12:56 available online: insectscience.org/12.56 Journal of Insect Science | www.insectscience.org 1 Journal of Insect Science: Vol. 12 | Article 56 Tan and Nishida 1. Introduction ME has been successfully used in: a) fruit fly surveys (Tan and Lee 1982) and quarantine Plants produce a huge array of chemicals, detection (see reviews by Metcalf and Metcalf numbering tens of thousands, primarily for 1992; Vargas et al.