Classical Weed Biological Control Outcomes: a Catalogue-Based Analysis of Success Rates and Their Correlates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 1 DNA Barcodes Reveal Deeply Neglected Diversity and Numerous
Page 1 of 57 1 DNA barcodes reveal deeply neglected diversity and numerous invasions of micromoths in 2 Madagascar 3 4 5 Carlos Lopez-Vaamonde1,2, Lucas Sire2, Bruno Rasmussen2, Rodolphe Rougerie3, 6 Christian Wieser4, Allaoui Ahamadi Allaoui 5, Joël Minet3, Jeremy R. deWaard6, Thibaud 7 Decaëns7, David C. Lees8 8 9 1 INRA, UR633, Zoologie Forestière, F- 45075 Orléans, France. 10 2 Institut de Recherche sur la Biologie de l’Insecte, UMR 7261 CNRS Université de Tours, UFR 11 Sciences et Techniques, Tours, France. 12 3Institut de Systématique Evolution Biodiversité (ISYEB), Muséum national d'Histoire naturelle, 13 CNRS, Sorbonne Université, EPHE, 57 rue Cuvier, CP 50, 75005 Paris, France. 14 4 Landesmuseum für Kärnten, Abteilung Zoologie, Museumgasse 2, 9020 Klagenfurt, Austria 15 5 Department of Entomology, University of Antananarivo, Antananarivo 101, Madagascar 16 6 Centre for Biodiversity Genomics, University of Guelph, 50 Stone Road E., Guelph, ON 17 N1G2W1, Canada 18 7Centre d'Ecologie Fonctionnelle et Evolutive (CEFE UMR 5175, CNRS–Université de Genome Downloaded from www.nrcresearchpress.com by UNIV GUELPH on 10/03/18 19 Montpellier–Université Paul-Valéry Montpellier–EPHE), 1919 Route de Mende, F-34293 20 Montpellier, France. 21 8Department of Life Sciences, Natural History Museum, Cromwell Road, SW7 5BD, UK. 22 23 24 Email for correspondence: [email protected] For personal use only. This Just-IN manuscript is the accepted prior to copy editing and page composition. It may differ from final official version of record. 1 Page 2 of 57 25 26 Abstract 27 Madagascar is a prime evolutionary hotspot globally, but its unique biodiversity is under threat, 28 essentially from anthropogenic disturbance. -

DNA Barcodes Reveal Deeply Neglected Diversity and Numerous Invasions of Micromoths in Madagascar
Genome DNA barcodes reveal deeply neglected diversity and numerous invasions of micromoths in Madagascar Journal: Genome Manuscript ID gen-2018-0065.R2 Manuscript Type: Article Date Submitted by the 17-Jul-2018 Author: Complete List of Authors: Lopez-Vaamonde, Carlos; Institut National de la Recherche Agronomique (INRA), ; Institut de Recherche sur la Biologie de l’Insecte (IRBI), Sire, Lucas; Institut de Recherche sur la Biologie de l’Insecte Rasmussen,Draft Bruno; Institut de Recherche sur la Biologie de l’Insecte Rougerie, Rodolphe; Institut Systématique, Evolution, Biodiversité (ISYEB), Wieser, Christian; Landesmuseum für Kärnten Ahamadi, Allaoui; University of Antananarivo, Department Entomology Minet, Joël; Institut de Systematique Evolution Biodiversite deWaard, Jeremy; Biodiversity Institute of Ontario, University of Guelph, Decaëns, Thibaud; Centre d'Ecologie Fonctionnelle et Evolutive (CEFE UMR 5175, CNRS–Université de Montpellier–Université Paul-Valéry Montpellier–EPHE), , CEFE UMR 5175 CNRS Lees, David; Natural History Museum London Keyword: Africa, invasive alien species, Lepidoptera, Malaise trap, plant pests Is the invited manuscript for consideration in a Special 7th International Barcode of Life Issue? : https://mc06.manuscriptcentral.com/genome-pubs Page 1 of 57 Genome 1 DNA barcodes reveal deeply neglected diversity and numerous invasions of micromoths in 2 Madagascar 3 4 5 Carlos Lopez-Vaamonde1,2, Lucas Sire2, Bruno Rasmussen2, Rodolphe Rougerie3, 6 Christian Wieser4, Allaoui Ahamadi Allaoui 5, Joël Minet3, Jeremy R. deWaard6, Thibaud 7 Decaëns7, David C. Lees8 8 9 1 INRA, UR633, Zoologie Forestière, F- 45075 Orléans, France. 10 2 Institut de Recherche sur la Biologie de l’Insecte, UMR 7261 CNRS Université de Tours, UFR 11 Sciences et Techniques, Tours, France. -

Final Report
Final Report Final pre-release investigations of the gorse thrips (Sericothrips staphylinus) as a biocontrol agent for gorse (Ulex europaeus) in North America Date: August 31, 2012 Award Number: 10-CA-11420004-184 Report Period: June 1, 2010– May 31, 2012 Project Period: June 1, 2010– May 31, 2012 Recipient: Oregon State University Recipient Contact Person: Fritzi Grevstad Principal Investigator/ Project Director: Fritzi Grevstad Introduction Gorse (Ulex europaeus) is an environmental weed classified as noxious in the states of Washington, Oregon, California, and Hawaii. A classical biological control program has been applied in Hawaii with the introduction of 4 gorse-feeding arthropods, but only two of these (a mite and a seed weevil) have been introduced to the mainland U.S. The two insects that have not yet been introduced include the gorse thrips, Sericothips staphylinus (Thysanoptera: Thripidae), and the moth Agonopterix umbellana (Lepidoptera: Oecophoridae). With prior support from the U.S. Forest Service (joint venture agreement # 07-JV-281), we were able to complete host specificity testing of S. staphylinus on 44 North American plant species that were on the original test plant list. However, following review of the proposed Test Plant List, the Technical Advisory Group on Biocontrol of Weeds (TAG) recommended that we include an additional 18 plant species for testing. In this report, we present host specificity testing and related objectives necessary to bring the program to the implementation stage. Objectives (1) Acquire and grow the additional 18 species of plants recommended by the TAG. (2) Complete host specificity trials for the gorse thrips on the 18 plant species. -

A New Leaf-Mining Moth from New Zealand, Sabulopteryx Botanica Sp
A peer-reviewed open-access journal ZooKeys 865: 39–65A new (2019) leaf-mining moth from New Zealand, Sabulopteryx botanica sp. nov. 39 doi: 10.3897/zookeys.865.34265 MONOGRAPH http://zookeys.pensoft.net Launched to accelerate biodiversity research A new leaf-mining moth from New Zealand, Sabulopteryx botanica sp. nov. (Lepidoptera, Gracillariidae, Gracillariinae), feeding on the rare endemic shrub Teucrium parvifolium (Lamiaceae), with a revised checklist of New Zealand Gracillariidae Robert J.B. Hoare1, Brian H. Patrick2, Thomas R. Buckley1,3 1 New Zealand Arthropod Collection (NZAC), Manaaki Whenua–Landcare Research, Private Bag 92170, Auc- kland, New Zealand 2 Wildlands Consultants Ltd, PO Box 9276, Tower Junction, Christchurch 8149, New Ze- aland 3 School of Biological Sciences, The University of Auckland, Private Bag 92019, Auckland, New Zealand Corresponding author: Robert J.B. Hoare ([email protected]) Academic editor: E. van Nieukerken | Received 4 March 2019 | Accepted 3 May 2019 | Published 22 Jul 2019 http://zoobank.org/C1E51F7F-B5DF-4808-9C80-73A10D5746CD Citation: Hoare RJB, Patrick BH, Buckley TR (2019) A new leaf-mining moth from New Zealand, Sabulopteryx botanica sp. nov. (Lepidoptera, Gracillariidae, Gracillariinae), feeding on the rare endemic shrub Teucrium parvifolium (Lamiaceae), with a revised checklist of New Zealand Gracillariidae. ZooKeys 965: 39–65. https://doi.org/10.3897/ zookeys.865.34265 Abstract Sabulopteryx botanica Hoare & Patrick, sp. nov. (Lepidoptera, Gracillariidae, Gracillariinae) is described as a new species from New Zealand. It is regarded as endemic, and represents the first record of its genus from the southern hemisphere. Though diverging in some morphological features from previously de- scribed species, it is placed in genus Sabulopteryx Triberti, based on wing venation, abdominal characters, male and female genitalia and hostplant choice; this placement is supported by phylogenetic analysis based on the COI mitochondrial gene. -

T1)E Bedford,1)Ire Naturaii,T 45
T1)e Bedford,1)ire NaturaIi,t 45 Journal for the year 1990 Bedfordshire Natural History Society 1991 'ISSN 0951 8959 I BEDFORDSHffiE NATURAL HISTORY SOCIETY 1991 Chairman: Mr D. Anderson, 88 Eastmoor Park, Harpenden, Herts ALS 1BP Honorary Secretary: Mr M.C. Williams, 2 Ive! Close, Barton-le-Clay, Bedford MK4S 4NT Honorary Treasurer: MrJ.D. Burchmore, 91 Sundon Road, Harlington, Dunstable, Beds LUS 6LW Honorary Editor (Bedfordshire Naturalist): Mr C.R. Boon, 7 Duck End Lane, Maulden, Bedford MK4S 2DL Honorary Membership Secretary: Mrs M.]. Sheridan, 28 Chestnut Hill, Linslade, Leighton Buzzard, Beds LU7 7TR Honorary Scientific Committee Secretary: Miss R.A. Brind, 46 Mallard Hill, Bedford MK41 7QS Council (in addition to the above): Dr A. Aldhous MrS. Cham DrP. Hyman DrD. Allen MsJ. Childs Dr P. Madgett MrC. Baker Mr W. Drayton MrP. Soper Honorary Editor (Muntjac): Ms C. Aldridge, 9 Cowper Court, Markyate, Herts AL3 8HR Committees appointed by Council: Finance: Mr]. Burchmore (Sec.), MrD. Anderson, Miss R. Brind, Mrs M. Sheridan, Mr P. Wilkinson, Mr M. Williams. Scientific: Miss R. Brind (Sec.), Mr C. Boon, Dr G. Bellamy, Mr S. Cham, Miss A. Day, DrP. Hyman, MrJ. Knowles, MrD. Kramer, DrB. Nau, MrE. Newman, Mr A. Outen, MrP. Trodd. Development: Mrs A. Adams (Sec.), MrJ. Adams (Chairman), Ms C. Aldridge (Deputy Chairman), Mrs B. Chandler, Mr M. Chandler, Ms]. Childs, Mr A. Dickens, MrsJ. Dickens, Mr P. Soper. Programme: MrJ. Adams, Mr C. Baker, MrD. Green, MrD. Rands, Mrs M. Sheridan. Trustees (appointed under Rule 13): Mr M. Chandler, Mr D. Green, Mrs B. -

Biological Control of Miconia Calvescens with a Suite of Insect Herbivores from Costa Rica and Brazil
Biological control of Miconia calvescens with a suite of insect herbivores from Costa Rica and Brazil F.R. Badenes-Perez,1,2 M.A. Alfaro-Alpizar,3 A. Castillo-Castillo3 and M.T. Johnson4 Summary Miconia calvescens DC. (Melastomataceae) is an invasive tree considered the most serious threat to the natural ecosystems of Hawaii and other Pacific islands. We evaluated nine species of natural enemies that feed on inflorescences or leaves ofM. calvescens for their potential as biological control agents, comparing their impact on the target plant, host specificity, and vulnerability to biotic inter- ference. Among herbivores attacking reproductive structures of M. calvescens, a fruit-galling wasp from Brazil, Allorhogas sp. (Hymenoptera: Braconidae), and a flower- and fruit-feeding moth from Costa Rica, Mompha sp. (Lepidoptera: Momphidae), were the most promising agents studied. The sawflyAtomacera petroa Smith (Hymenoptera: Argidae) from Brazil was thought to have the highest potential among the defoliators evaluated. Keywords: herbivory, host specificity, biotic interference. Introduction ductive structures may be necessary to achieve effec- tive biological control. Miconia calvescens DC. (Melastomataceae) is a small Several insects and pathogens have been identified tree native to Central and South America that is consid- as potential agents in surveys conducted in the native ered a serious threat to natural ecosystems in Hawaii range of M. calvescens in Brazil and Costa Rica by and other Pacific islands because of its ability to invade Johnson (unpublished data) and others (Burkhart, 1995; native forests (Medeiros et al., 1997). Its devastating Barreto et al., 2005; Picanço et al., 2005). In the inter- effects are most evident in Tahiti, where it has displaced est of avoiding unnecessary introductions and making over 65% of the native forest and threatens many en- efficient use of limited resources to evaluate potential demic species (Meyer and Florence, 1996). -
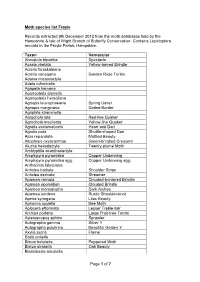
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

Prickly Acacia in Queensland Is Generally Accepted to Be Acacia Nilotica Subspecies Indica
PRICKLY ACACIA Prickly acacia (Acacia nilotica) in Queensland PEST STATUS REVIEW SERIES - LAND PROTECTION BRANCH Edited by A.P. Mackey Assessment team: M. Barker W. Dorney P. James P. Jeffrey N. March J. Marohasy D. Panetta Acknowledgements This assessment draws heavily on reports by P. Jeffrey and M. Marker and valuable discussions with J. Carter. Cover and contents design: Grant Flockhart and Sonia Jordan Photographic credits: Natural Resources and Mines staff ISBN 0 7242 6969 X Published by the Department of Natural Resources and Mines, Qld. Information in this document may be copied for personal use or published for educational purposes, provided that any extracts are fully acknowledged. Land Protection Department of Natural Resources and Mines Locked Bag 40, Coorparoo Delivery Centre, Q, 4151 Contents 1.0 Summary.....................................................................................................1 2.0 Taxonomic Status. .....................................................................................2 3.0 History of Introduction and Spread. .........................................................3 4.0 Current and Predicted Potential Distribution. .........................................4 5.0 Estimates of Current and Potential Impact..............................................7 5.1 Impact on Primary Industry............................................................................ 7 5.2 Control Costs. ................................................................................................ 7 5.3 Environmental -

Micro Moths on Great Cumbrae Island (Vc100)
The Glasgow Naturalist (online 2017) Volume 26, xx-xx Micro moths on Great Cumbrae Island (vc100) P. G. Moore 32 Marine Parade, Millport, Isle of Cumbrae KA28 0EF E-mail: [email protected] ABSTRACT Forsythia sp. Behind the office is a large mature Few previous records exist for miCro-moths from black mulberry tree (Morus nigra) and to one side is vC100. Data are presented from the first year-round a tall privet hedge (Ligustrum ovalifolium). To the moth-trapping exerCise accomplished on Great rear of my property is a wooded escarpment with Cumbrae Island; one of the least studied of the old-growth ash (Fraxinus excelsior) frequently ivy- Clyde Isles (vC100). Data from a Skinner-type light- Covered (Hedera helix), sycamore (Acer trap, supplemented by Collection of leaf mines from pseudoplatanus) and rowan (Sorbus aucuparia), local trees, revealed the presence of 71 species of with an undergrowth of hawthorn (Crataegus miCro moths, representing 20 new records for the monogyna), wild garliC (Allium ursinum), nettle vice-County. (Urtica dioica), bracken (Pteridium aquilinum) and bramble (Rubus fructicosus). Rhind (1988) detailed INTRODUCTION the vasCular plants found on Great Cumbrae Island The extensive nineteenth-century list of between 1985 and 1987 and delineated the history Lepidoptera in the 1901 handbook on the natural of the island's botanical investigations. Leaves of history of Glasgow and the West of SCotland issued brambles in my garden, beech trees (Fagus for the Glasgow meeting of the British AssoCiation sylvatica) and hazel (Corylus avellana) at other for the Advancement of SCience (Elliot et al., 1901) locations on the island (respectively Craiglea Wood inCluded few Cumbrae records. -

Additions, Deletions and Corrections to An
Bulletin of the Irish Biogeographical Society No. 36 (2012) ADDITIONS, DELETIONS AND CORRECTIONS TO AN ANNOTATED CHECKLIST OF THE IRISH BUTTERFLIES AND MOTHS (LEPIDOPTERA) WITH A CONCISE CHECKLIST OF IRISH SPECIES AND ELACHISTA BIATOMELLA (STAINTON, 1848) NEW TO IRELAND K. G. M. Bond1 and J. P. O’Connor2 1Department of Zoology and Animal Ecology, School of BEES, University College Cork, Distillery Fields, North Mall, Cork, Ireland. e-mail: <[email protected]> 2Emeritus Entomologist, National Museum of Ireland, Kildare Street, Dublin 2, Ireland. Abstract Additions, deletions and corrections are made to the Irish checklist of butterflies and moths (Lepidoptera). Elachista biatomella (Stainton, 1848) is added to the Irish list. The total number of confirmed Irish species of Lepidoptera now stands at 1480. Key words: Lepidoptera, additions, deletions, corrections, Irish list, Elachista biatomella Introduction Bond, Nash and O’Connor (2006) provided a checklist of the Irish Lepidoptera. Since its publication, many new discoveries have been made and are reported here. In addition, several deletions have been made. A concise and updated checklist is provided. The following abbreviations are used in the text: BM(NH) – The Natural History Museum, London; NMINH – National Museum of Ireland, Natural History, Dublin. The total number of confirmed Irish species now stands at 1480, an addition of 68 since Bond et al. (2006). Taxonomic arrangement As a result of recent systematic research, it has been necessary to replace the arrangement familiar to British and Irish Lepidopterists by the Fauna Europaea [FE] system used by Karsholt 60 Bulletin of the Irish Biogeographical Society No. 36 (2012) and Razowski, which is widely used in continental Europe. -

Über Die Puppen Der Mittel- Und Westeuropäischen Gracillariinae (Lepidoptera: Gracillariidae)
Über die Puppen der mittel- und westeuropäischen Gracillariinae (Lepidoptera: Gracillariidae) J. Patoöka PATOCKA, J., 2001. ON THE PUPAE OF THE CENTRAL AND WESTERN EUROPEAN GRACILLARIINAE (LEPIDOPTERA: GRACILLARIIDAE). - ENT. BER., AMST. 61 (11): 157-164. Abstract: Pupae of seven species of Central and Western European Gracillariinae (Lepidoptera: Gracillariidae) are de¬ scribed and illustrated. Diagnostic characteristics of the species are compared with those of similar taxa of this subfami¬ ly. Some biological data are added. Institut für Waldökologie der SAW, Stürova 2, 960 53 Zvolen, Republik Slowakei. Die vorliegende Arbeit stellt einen Nachtrag Raupen an Asteraceae spp. In Mitteleuropa zu den Arbeiten von Patocka (1992a, 1992b) mit einer, in Westeuropa mit keiner Art ver¬ und Patocka & Zach (1995) vor. Sie behandelt treten. die Puppen der sieben von dem Autor bisher Bemerkung: Bei der Bestimmung dieser nicht untersuchten Arten der mittel- und Gattung mit Hilfe der Gattungstabelle der westeuropäischen Gracillariinae. Eine Cha¬ Gracillariidae in Patocka (1992 a) kommt man rakteristik der Lepidopteren-Puppen und Be¬ zum Punkt 11, Gattungen: Acrocercops Wal- stimmungstabelle deren mitteleuropäischer lengren und Parectopa Clemens. Von den Überfamilien und Familien anhand der Pup¬ beiden unterscheidet sich Aristaea durch die penmerkmale bringt Patocka (1999). Das frontale Richtung des Kopffortsatzes und System und die Nomenklatur folgt Karsholt & dadurch, daß der Kopf kaudal davon nur Razowski (1996) und berücksichtigt auch schwach gewölbt ist (Abb. 2-4). Von Parec¬ Leraut (1997) und Lastuvka (1998). Das topa ferner durch die Abwesenheit je einer untersuchte Material stammt aus der Samm¬ starken Kopfborste (Abb. 3), von Acrocercops lung des Verfassers, aus dem Museum für Na¬ und Dialectica Clemens durch das in der turkunde Berlin und der Zoologischen Staats¬ Mitte nur mittelstark verjüngte Pronotum sammlung München. -

Federal Register / Vol. 60, No. 240 / Thursday, December 14, 1995 / Notices
64140 Federal Register / Vol. 60, No. 240 / Thursday, December 14, 1995 / Notices In accordance with applicable into the environment of the organisms relevant literature, provide the public regulations, APHIS has received listed below after concluding that their with documentation of APHIS' review applications for permits for the release release in accordance with conditions and analysis of the environmental into the environment of nonindigenous on the permits will not present a risk of impact and plant pest risk associated biological control agents. In the course the introduction or dissemination of with releasing the biological control of reviewing each permit application, plant pests within the United States and agents into the environment. APHIS assessed the plant pest risk will not have a significant impact on the Environmental assessments and posed by each organism and the impact quality of the human environment. The findings of no significant impact have on the environment of releasing each environmental assessments and findings been prepared by APHIS relative to the organism under the conditions of no significant impact, which are issuance of permits for the release into described in the permit application. based on data submitted by the the environment of the following APHIS has issued permits for the release applicant and on a review of other biological control agents: Date of find- ing of no Organism Title of environmental assessment significant impact Cirrospilus quadristriatus (Subba Rao and ``Field Release of a Nonindigenous Wasp (Cirrospilus quadristriatus (Subba Rao and 7/25/94. Ramamani). Ramamani)) for Biological Control of Citrus Leafminer (Phyllocnistis citrella Stainton)'' (July 1994). Anaphes nitens (Girault) ............................