Images Paediatr Cardiol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surgical Instruments �������������������������������������������
SURGICAL INSTRUMENTS Claudia Gherman, ăCiocan, Ovidiu Fabian Learning objectives What you should know The main types of surgical instruments The main instruments used for cutting tissues The main instruments used for tissue manipulation The main instruments used for exposure (retractors) The main instruments used for suturing The functioning principle of electrocautery devices The main laparoscopic instruments What you should do Recognize the main surgical instruments Attach a scalpel blade to a handle/remove it from the handle Hand a scalpel to another person correctly Perform an incision Handle scissors (hold them correctly, cut under visual control, hand scissors to another person) Handle a self-retaining forceps (hold it correctly, grip the tissue, close and open the forceps, hand it to another person) Hold a retractor correctly Hold, close/open and hand over a needle holder correctly Recognize a suturing needle; recognize a sharp needle and an intestinal needle; find on the needle and suture package the main information about the needle Classification In order to perform surgery, the surgical team needs a number of surgical instruments. Each of the thousands of instruments used is designed for a specific function. They can be classified depending on use as follows: Cutting instruments Instruments for tissue grasping and manipulation Instruments for tissue exposure Suturing instruments Hybrid instruments Endoscopic instruments Cutting instruments Scalpels: consist of a handle and a blade; the handle is made of metal (reusable) or plastic (disposable); blades are disposable, of various shapes and sizes. The top of the scalpel handle has a special part, with a groove that allows its sliding into the blade slot and securing of the blade in position. -

Surgery Instrumnts Khaled Khalilia Group 7
Surgery Instrumnts khaled khalilia Group 7 Scalpel handle blade +blade scalpel blade disposable fixed blade knife (Péan - Hand-grip : This grip is best for initial incisions and larger cuts. - Pen-grip : used for more precise cuts with smaller blades. - Changing Blade with Hemostat Liston Charrière Saw AmputationAmputati knife on knife Gigli Saw . a flexible wire saw used by surgeons for bone cutting .A gigli saw is used mainly for amputation surgeries. is the removal of a body extremity by trauma, prolonged constriction, or surgery. Scissors: here are two types of scissors used in surgeries.( zirconia/ ceramic,/ nitinol /titanium) . Ring scissors look much like standard utility scissors with two finger loops. Spring scissors are small scissors used mostly in eye surgery or microsurgery . Bandage scissors: Bandage scissors are angled tip scissors. helps in cutting bandages without gouging the skin. To size bandages and dressings. To cut through medical gauze. To cut through bandages already in place. Tenotomy Scissors: used to perform delicate surgery. used to cut small tissues They can be straight or curved, and blunt or sharp, depending upon necessity. operations in ophthalmic surgery or in neurosurgery. 10 c”m Metzenbaum scissors: designed for cutting delicate tissue come in variable lengths and have a relatively long shank-to-blade ratio blades can be curved or straight. the most commonly used scissors for cutting tissue. Use: ental, obstetrical, gynecological, dermatological, ophthalmological. Metzenbaum scissors Bandage scissors Tenotomy scissors Surgical scissors Forceps: Without teeth With teeth Dissecting forceps (Anatomical) With teeth: for tougher(hart) tissue: Fascia,Skin Without teeth: (atraumatic): for delicate tissues (empfindlich): Bowel Vessels. -

49 How to Grasp the Needle with Needle
Basic Instrument Use Video Transcript How to Grasp the Needle with Needle Holders Placing a stitch requires a sequence of carefully planned steps. Each step, if performed incorrectly, contributes to the loss of precious operative time. Skilled surgeons do not necessarily suture with rapid hand motions, but use deliberate, disciplined steps that eliminate nonproductive motions during suturing. Novice surgeons must practice handling the needle holder and placing sutures in order to learn how to perform this technique pr operly and efficiently. In this video, we will examine the first step of placing a stitch, how to grasp the needle within the jaws of the needle holder. To grasp the needle in the needle holder, release the ratchet and open the jaws. The needle is positioned between the jaws, at the tip of the instrument, and is almost always held perpendicular to the axis of the jaws. This standard position allows for good needle stability and helps prevent bending. When the needle is held in this manner, the needle can be driven through tissue by merely rotating the holder on its axis. This minimizes the amount of motion needed to drive the needle, using less space to complete the stitch. When the needle is placed obliquely within the jaws, the handle of the holders must move through a wider arc to follow the curve of the needle through the tissue. There are few surgical situations in which oblique placement of the needle is required. When grasping the needle with needle holders, you will also need to plan where along the curve of the needle it should be grasped. -

Instruments 449-478 4/3/06 10:42 AM Page 449
Instruments_449-478 4/3/06 10:42 AM Page 449 Neuro Hammers & Diagnostic ADC® NEUROLOGICAL HAMMERS Four of the most popular hammers for diagnosis of neurological function. 369110105375 Buck Hammer, 7 1/4˝, Chrome Plated Handle w/2 sided rubber head, Handle Conceals “screw-in” Brush, Needle Contained Within The Head 369310105374 Taylor Hammer, 7 1/2˝, Chrome Handle w/triangular rubber head, Orange 3693BK10141795 Taylor Hammer, 7 1/2˝, Chrome Handle w/triangular rubber head, Black 3693DG10141796 Taylor Hammer, 7 1/2˝, Chrome Handle w/triangular rubber head, Dark Green 3693RB10141797 Taylor Hammer, 7 1/2˝, Chrome Handle w/triangular rubber head, ADC® TUNING FORKS Royal Blue 369510105372 Wartenberg Pinwheel, 7 1/2˝, Stainless Steel Handle w/textured grip, Non magnetic, corrosion resistant aluminum alloy construction weighs 1/3 of Rotating Spur comparable steel tuning forks. Produced from 3/8˝ x 1˝ bar stock for superior 369710105373 Babinski Hammer, 8 1/2˝, Octagonal Stainless Steel Handle w/concealed performance and consistent frequency accuracy. Extra long 2˝ handle of turned needle, Rubber Head smooth aluminum to facilitate bone conduction tests. 50012810105366 Tuning Fork w/fixed weight, 128cps Frequency 50025610105367 Tuning Fork w/fixed weight, 256cps Frequency 50051210105368 Tuning Fork w/o weight, 512cps Frequency 50102410105369 Tuning Fork w/o weight, 1024cps Frequency 50204810105370 Tuning Fork w/o weight, 2048cps Frequency 50409610105371 Tuning Fork w/o weight, 4096cps Frequency 1-200 1-220 MILTEX HAMMERS 1-20010090643 Taylor Percussion -
Fine Surgical Instruments for Research™ Hsin-Yi Road, Sec
INTERNATIONAL Scissors, Bone Instruments Fine Science Tools Inc. & Scalpels 202-277 Mountain Highway pages 3–61 North Vancouver, British Columbia Canada V7J 3T6 Telephone 800-665-5355 / 604-980-2481 Fax 800-665-4544 / 604-987-3299 E-Mail [email protected] Web finescience.ca Fine Science Tools (USA) Inc. 373-G Vintage Park Drive F Forceps & Hemostats Foster City, California 94404-1139 I pages 63–97 Telephone 800-521-2109 / 650-349-1636 N Fax 800-523-2109 / 650-349-3729 E E-Mail [email protected] Web finescience.com S C I E Fine Science Tools GmbH N Vangerowstraße 14 C 69115 Heidelberg Germany E Telephone +49 (0) 62 21 - 90 50 50 Fax +49 (0) 62 21 - 90 50 590 T Probes, Needle Holders, O E-Mail [email protected] Thread, Retractors & Clamps Web finescience.de O pages 99–133 L S InterFocus Ltd. Pentagon Business Park Cambridge Road Linton, Cambridge CB21 4NN C United Kingdom A Telephone +44 (0)1223 894833 T Fax +44 (0)1223 894235 A Surgical & Laboratory E-Mail [email protected] L Accessories Web surgicaltools.co.uk O pages 135–161 G 2 Muromachi Kikai Co., Ltd. 0 4-2-12, Nihonbashi-Muromachi 1 Chuo-ku 4 Tokyo 103-0022 Japan Telephone (03) 3241-2444 Fax (03) 3241-2940 E-Mail [email protected] Student Quality Instruments Web muromachi.com pages 163–167 Proserv Instruments Co., Ltd. 7F-2, No. 413 Fine Surgical Instruments for Research™ Hsin-Yi Road, Sec. 4 Taipei, Taiwan R.O.C. Telephone (02) 27230455 Fax (02) 27230799 2014 E-Mail [email protected] Web proserv.com.tw TABLE OF CONTENTS | CATALOG 2014 Scissors 3 – 37 Spring 3 – 14 WE PROUDLY STOCK Fine 15 – 30 Surgical 31 – 37 Bone Instruments 38 – 51 DUMONT® Rongeurs 38 – 41 A selection of over 50 of the most popular Cutters 42 – 49 Other Bone Instruments 50 Dumont forceps are offered in this catalog. -

THERMAL SCALPEL SYSTEM MODEL PRECISION 8400 (P8400) HANDLE Part Number 7013-8050
THERMAL SCALPEL SYSTEM MODEL PRECISION 8400 (P8400) HANDLE Part Number 7013-8050 For use with the Hemostatix Thermal Scalpel System Model P8400 Controller OPERATING MANUAL INTRODUCTION HEMOSTATIX THERMAL SCALPEL BLADES are similar in size, shape, and sharpness to traditional steel scalpel blades; however, Hemostatix blades can be heated to a user- selected temperature appropriate for sealing small vessels as they are cut. To cut and simultaneously seal blood vessels effectively with minimum tissue damage, the sharp steel cutting edge of a heated scalpel blade must be uniformly maintained at the desired temperature within narrow limits. The Hemostatix Thermal Scalpel System utilizes micro-circuitry incorporated within the blade itself to maintain the cutting edge temperature within the necessary tolerance, selectively delivering additional thermal energy only to those regions of the blade using heat due to tissue contact. By so doing, the Hemostatix Thermal Scalpel System automatically compensates for the varying degrees of heat loss that occur during surgical procedures (depending on the type of tissue being incised and the rate at which cutting is carried out), maintaining the cutting edge in the desired temperature range. In contrast with electrosurgical devices, the Hemostatix Thermal Scalpel System passes no electrical current through the patient, and there is no sparking or electrical arcing to the tissue. Electrosurgical devices “cut” and/or cauterize using electrical currents which pass through the patient vaporizing tissue at the point of contact and generating heat and tissue damage down the path of the electrical current. The Hemostatix Thermal Scalpel System cuts tissue with a sharpened steel edge, like a conventional cold-steel scalpel blade, and simultaneously seals blood vessels using heat thermally conducted to the tissue from an elevated-temperature blade which is electrically insulated from the patient. -

Federal Chargemaster Price Transparency Edgewood (2).Xlsx
EDGEWOOD SURGICAL HOSPITAL CHARGES Federal reporting rules require hospitals to maintain a catalog of thousands of procedure codes, code descriptions and list prices in a complex accounting tool, known as the hospital chargemaster. The prices listed on the chargemaster do not reflect what patients will ultimately pay as insurance companies negotiate discounts on the list prices. In addition, co-pays, co-insurance and deductibles can also bring additional discounts before a final charge is determined. To get an accurate estimate of what your out of pocket expenses will be, contact us at (724) 646-0400, Monday through Friday, from 8 a.m. – 4:30 p.m. Chg Code Description Chg Amt 1 NF-HUMULIN R INJ SOLN 100U/1ML $61.61 99077 EXTENDED RECOVERY ROOM PER MINUTE $15.00 99078 OBSERVATION 1-4 HOURS $550.00 99079 OBSERVATION >5 HOURS **EACH** $15.00 99085 OR TIME PER MINUTE COMPLEX (>3 STAFF) $197.00 99086 OR TIME PER MINUTE MAJOR (3 STAFF) $136.00 99087 OR TIME PER MINUTE MINOR (1-2 STAFF) $93.00 99088 SURGICAL NEUROMONITORING $1,350.00 99089 SURGICAL EYE LASER $1,743.00 99090 PAIN MANAGEMENT PER MINUTE $187.00 99091 OR TIME PER MINUTE ADDITIONAL STAFF $1.00 99092 FORCE TRIAD RENTAL $350.00 99093 YAG LASER CHARGE $1,182.00 99094 PAIN MANAGEMENT PER MINUTE RF $326.00 99100 CONS SEDATION (SAME DOC) <5YR 30-MIN $302.00 99101 CONS SEDATION (SAME DOC) <5YR 30-MIN $302.00 99102 CONS SEDATION (SAME DOC) ADD'S 15-MIN $151.00 99103 CONS SEDATION (DIFF DOC) <5YR 30-MIN $302.00 99104 CONS SEDATION (DIFF DOC) >5 YR 30-MIN $302.00 99105 CONS SEDATION (DIFF -
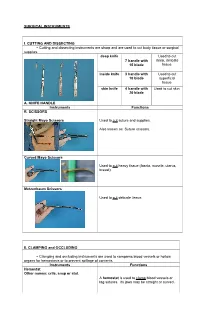
Surgical Instruments
SURGICAL INSTRUMENTS I. CUTTING AND DISSECTING ~ Cutting and dissecting instruments are sharp and are used to cut body tissue or surgical supplies. deep knife Used to cut 7 handle with deep, delicate 15 blade tissue. inside knife 3 handle with Used to cut 10 blade superficial tissue. skin knife 4 handle with Used to cut skin. 20 blade A. KNIFE HANDLE Instruments Functions B. SCISSORS Straight Mayo Scissors Used to cut suture and supplies. Also known as: Suture scissors. Curved Mayo Scissors Used to cut heavy tissue (fascia, muscle, uterus, breast). Metzenbaum Scissors Used to cut delicate tissue. II. CLAMPING and OCCLUDING ~ Clamping and occluding instruments are used to compress blood vessels or hollow organs for hemostasis or to prevent spillage of contents. Instruments Functions Hemostat Other names: crile, snap or stat. A hemostat is used to clamp blood vessels or tag sutures. Its jaws may be straight or curved. Hemostat, Mosquito (left to right) A mosquito is used to clamp small blood vessels. Its jaws may be straight or curved. Kelly, Hemostat, Mosquito (left to right) Other names: Rochester Pean A Kelly is used to clamp larger vessels and tissue Burlisher Other names: Schnidt tonsil forcep, Adson forcep. A burlisher is used to clamp deep blood vessels. Burlishers have two closed finger rings. Burlishers with an open finger ring are called tonsil hemostats. Right Angle Other names: Mixter. A right angle is used to clamp hard-to-reach vessels and to place sutures behind or around a vessel. A right angle with a suture attached is called a "tie on a passer." Hemoclip applier with hemoclips A hemoclip applier with hemoclips applies metal clips onto blood vessels and ducts which will remain occluded. -

A Novel Right Ventricular Volume and Pressure Loaded Piglet Heart Model for the Study of Tricuspid Valve Function
Materials List for A Novel Right Ventricular Volume and Pressure Loaded Piglet Heart Model for the Study of Tricuspid Valve Function. Lily Q. Lin1, Sanaz Hatami2, James Yashu Coe1, Timothy M. Colen1, Consolato Sergi3, Richard Thompson4, Elena S. Di Martino5, Walter Herzog6, Ziad Abu Sara6, Darren H. Freed2, Nee Scze Khoo1 1Division of Pediatric Cardiology, Department of Pediatrics, University of Alberta 2Department of Surgery, University of Alberta 3Department of Laboratory Medicine and Pathology, University of Alberta 4Department of Biomedical Engineering, University of Alberta 5Department of Civil Engineering, University of Calgary 6Faculty of Kinesiology, University of Calgary Corresponding Author Citation Lily Q. Lin Lin, L.Q., Hatami, S., Coe, J.Y., Colen, T.M., Sergi, C., Thompson, R., Di Martino, E.S., [email protected] Herzog, W., Abu Sara, Z., Freed, D.H., Khoo, N.S. A Novel Right Ventricular Volume and Pressure Loaded Piglet Heart Model for the Study of Tricuspid Valve Function.. J. Vis. Exp. (), e61251, doi:10.3791/61251 (2020). Date Published DOI URL July 28, 2020 10.3791/61251 jove.com/video/61251 Materials Name Company Catalog Number Comments Drugs 1% lidocaine spray WDDC 103365 Lidodan 30 mL atropine sodium injection WDDC/Rafter 8 Products 0.5 mg/mL bupivacaine WDDC/Sterimax 5 mg/mL buprenorphine HCl slow release Chiron Compounding Pharmacy 1 mg/mL injection buprenorphine regular WDDC/Champion Alstoe 121378 Vetergesic 0.3 mg/mL cefazolin WDDC/Fresenius Kabi 102016 1 g/vial cephalexin capsule WDDC/Novopharm Novo-Lexin -

Clinic and Practice-Based Products Catalog
Premier Products for Women’s Health Care Clinic and Practice-Based Products Contraception Cervical Cryosurgery Hysteroscopy Ophthalmic Cryosurgery Endometrial Ablation Pessaries Urodynamics Obstetrical Care Colposcopy Fertility and Family Planning Gynecological Specialty Instruments General Examination: Diagnostic Disposables Endometrial Samplers Sono HSG LEEP Delivering on the Promise to Help Clinicians Provide Better Health Care for Women Our mission is to enable physicians to provide increasingly more efficient and effective health care for women. We leverage our expertise in identifying and developing a steady stream of exceptional products that keep Ob/Gyns and others directly involved in caring for women, at the forefront of women’s health care. An Expansive Portfolio of Trusted Brands And Services Since our founding in 1990, CooperSurgical has researched, developed and manufactured a wide range of trusted brands that have advanced the standard of women’s health care. Our products are used by physicians and health care professionals in hospitals and their practices for a wide variety of procedures, from basic annual gynecological exams to complex surgical procedures. As a full-service R&D and manufacturing company, we are committed to: • Improved patient outcomes and satisfaction • The highest quality and safety standards in the industry • Clinically proven technologies that provide ease of use • Technical support of our equipment – 20+ years of experience • Innovative solutions that reduce procedural costs • Annual support of medical professional educational organizations • Educational and in-service support of our products • Personalized service and a commitment to your satisfaction by educated professional representatives We appreciate the opportunity to partner with you to provide optimum health care for women. -
Stephens Instruments Catalog
cover front 7/5/07 11:19 AM Page 1 Volume II 09 catalog assembled v2 rev 01 9/2/09 10:48 AM Page 1 Stephens Instruments manufactures and distributes one of the largest selections of microsurgical instruments for Ophthalmology. We offer a complete line of stainless steel and titanium surgical instrumentation as well as a complete line of disposable knives. Our product range has been broadened to include products that have been developed through your experiences and advancements in technology. Stephens Instruments offers a repair service for all brands of ophthalmic surgical instruments. We are also at your service for any new ideas for instrumentation or modifications to existing instruments. Stephens Instruments manufactures it’s instruments utilizing the highest quality of German and U.S. stainless steel. All instruments are guaranteed against defects in material and/or workmanship. Illustrations in this catalogue generally represent actual size of instruments. Stephens Instruments reserves the right to make necessary changes to design, size, etc., as is required. All reusable instruments that are returned in unused condition within 30 days of invoice date will be fully refunded for any reason. Disposable, sterile instruments that have been opened may not be returned for a refund. We look forward to the opportunity to serve you. U.S.A. & Canada: 1-800-354-7848, 859-259-4924 or 859-259-4925 Fax: 859-259-4926 E-Mail: [email protected] www.stephensinst.com © 2000, 2007, 2009 Stephens Instruments. All rights reserved. No part of this document may be reproduced by any means. 09 catalog assembled v2 rev 01 9/2/09 10:48 AM Page 2 TABLE OF CONTENTS S1 Speculums, Retractors, Lid Clamp . -

Commonly Used Surgical Instruments
COMMON SURGICAL INSTRUMENTS The operating room contains a multitude of instruments fit for accomplishing a number of procedures. Note that this is not an exhaustive list of instruments, but rather some that you will encounter frequently. SCALPEL Used for initial incision and cutting tissue. Consists of a blade and a handle. Surgeons #10 Blade: Used primarily for #11 Blade: Used for making #15 Blade: Smaller version of often refer to the making large skin incisions, precise or sharply angled #10 blade used for making finer instrument by its blade e.g., in laparotomy. incisions. incisions. number. Pott’s Scissors: Fine scissors used for creating SCISSORS incisions in blood vessels. Used for cutting tissue, suture, or for Iris Scissors: Used Mayo Scissors: Heavy scissors Metzenbaum Scissors: Lighter dissection. Scissors for fine dissection available in multiple varieties. scissors used for cutting delicate can be straight or and cutting fine Straight scissors are used for tissue (e.g., heart) and for blunt curved, and may be suture. Originally cutting suture (“suture scissors”), dissection. Also called “Metz” in for ophthalmic used for cutting heavy while curved scissors are used practice. procedures, but or finer structures. for cutting heavy tissue (e.g., now serves fascia). multipurpose role. Bonney DeBakey Russian Forceps: Forceps: Forceps: Heavy Used for Used for Tissue Forceps: Non-toothed forceps atraumatic atraumatic FORCEPS forceps used for fine handling used for tissue tissue Also known as non- of tissue and traction during holding grasping grasping locking forceps, dissection. thick tissue during during grasping forceps, (e.g., fascial dissection. dissection. thumb forceps, or closure).