Cae331 513 Lecture10 Psychro
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vapour Absorption Refrigeration Systems Based on Ammonia- Water Pair
Lesson 17 Vapour Absorption Refrigeration Systems Based On Ammonia- Water Pair Version 1 ME, IIT Kharagpur 1 The specific objectives of this lesson are to: 1. Introduce ammonia-water systems (Section 17.1) 2. Explain the working principle of vapour absorption refrigeration systems based on ammonia-water (Section 17.2) 3. Explain the principle of rectification column and dephlegmator (Section 17.3) 4. Present the steady flow analysis of ammonia-water systems (Section 17.4) 5. Discuss the working principle of pumpless absorption refrigeration systems (Section 17.5) 6. Discuss briefly solar energy based sorption refrigeration systems (Section 17.6) 7. Compare compression systems with absorption systems (Section 17.7) At the end of the lecture, the student should be able to: 1. Draw the schematic of a ammonia-water based vapour absorption refrigeration system and explain its working principle 2. Explain the principle of rectification column and dephlegmator using temperature-concentration diagrams 3. Carry out steady flow analysis of absorption systems based on ammonia- water 4. Explain the working principle of Platen-Munter’s system 5. List solar energy driven sorption refrigeration systems 6. Compare vapour compression systems with vapour absorption systems 17.1. Introduction Vapour absorption refrigeration system based on ammonia-water is one of the oldest refrigeration systems. As mentioned earlier, in this system ammonia is used as refrigerant and water is used as absorbent. Since the boiling point temperature difference between ammonia and water is not very high, both ammonia and water are generated from the solution in the generator. Since presence of large amount of water in refrigerant circuit is detrimental to system performance, rectification of the generated vapour is carried out using a rectification column and a dephlegmator. -

Chapter 8 and 9 – Energy Balances
CBE2124, Levicky Chapter 8 and 9 – Energy Balances Reference States . Recall that enthalpy and internal energy are always defined relative to a reference state (Chapter 7). When solving energy balance problems, it is therefore necessary to define a reference state for each chemical species in the energy balance (the reference state may be predefined if a tabulated set of data is used such as the steam tables). Example . Suppose water vapor at 300 oC and 5 bar is chosen as a reference state at which Hˆ is defined to be zero. Relative to this state, what is the specific enthalpy of liquid water at 75 oC and 1 bar? What is the specific internal energy of liquid water at 75 oC and 1 bar? (Use Table B. 7). Calculating changes in enthalpy and internal energy. Hˆ and Uˆ are state functions , meaning that their values only depend on the state of the system, and not on the path taken to arrive at that state. IMPORTANT : Given a state A (as characterized by a set of variables such as pressure, temperature, composition) and a state B, the change in enthalpy of the system as it passes from A to B can be calculated along any path that leads from A to B, whether or not the path is the one actually followed. Example . 18 g of liquid water freezes to 18 g of ice while the temperature is held constant at 0 oC and the pressure is held constant at 1 atm. The enthalpy change for the process is measured to be ∆ Hˆ = - 6.01 kJ. -

Investigating Applicability of Evaporative Cooling Systems for Thermal Comfort of Poultry Birds in Pakistan
applied sciences Article Investigating Applicability of Evaporative Cooling Systems for Thermal Comfort of Poultry Birds in Pakistan Hafiz M. U. Raza 1, Hadeed Ashraf 1, Khawar Shahzad 1, Muhammad Sultan 1,* , Takahiko Miyazaki 2,3, Muhammad Usman 4,* , Redmond R. Shamshiri 5 , Yuguang Zhou 6 and Riaz Ahmad 6 1 Department of Agricultural Engineering, Bahauddin Zakariya University, Bosan Road, Multan 60800, Pakistan; [email protected] (H.M.U.R.); [email protected] (H.A.); [email protected] (K.S.) 2 Faculty of Engineering Sciences, Kyushu University, Kasuga-koen 6-1, Kasuga-shi, Fukuoka 816-8580, Japan; [email protected] 3 International Institute for Carbon-Neutral Energy Research (WPI-I2CNER), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan 4 Institute for Water Resources and Water Supply, Hamburg University of Technology, Am Schwarzenberg-Campus 3, 20173 Hamburg, Germany 5 Leibniz Institute for Agricultural Engineering and Bioeconomy, Max-Eyth-Allee 100, 14469 Potsdam-Bornim, Germany; [email protected] 6 Bioenergy and Environment Science & Technology Laboratory, College of Engineering, China Agricultural University, Beijing 100083, China; [email protected] (Y.Z.); [email protected] (R.A.) * Correspondence: [email protected] (M.S.); [email protected] (M.U.); Tel.: +92-333-610-8888 (M.S.); Fax: +92-61-9210298 (M.S.) Received: 4 June 2020; Accepted: 24 June 2020; Published: 28 June 2020 Abstract: In the 21st century, the poultry sector is a vital concern for the developing economies including Pakistan. The summer conditions of the city of Multan (Pakistan) are not comfortable for poultry birds. -
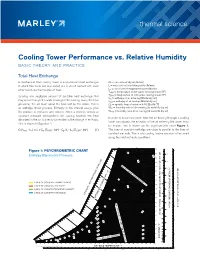
Thermal Science Cooling Tower Performance Vs. Relative Humidity
thermal science Cooling Tower Performance vs. Relative Humidity BASIC THEORY AND PRACTICE Total Heat Exchange A mechanical draft cooling tower is a specialized heat exchanger G = mass rate of dry air [lb/min] in which two fluids (air and water) are in direct contact with each L = mass rate of circulating water [lb/min] other to induce the transfer of heat. Le = mass rate of evaporated water [lb/min] THW = temperature of hot water entering tower [°F] Ignoring any negligible amount of sensible heat exchange that TCW = temperature of cold water leaving tower [°F] hin = enthalpy of air entering [Btu/lb/dry air] may occur through the walls (casing) of the cooling tower, the heat hout = enthalpy of air leaving [Btu/lb/dry air] gained by the air must equal the heat lost by the water. This is Cp = specific heat of water = 4.18 [Btu/lb-°F] an enthalpy driven process. Enthalpy is the internal energy plus Win = humidity ratio of air entering [lb water/lb dry air] the product of pressure and volume. When a process occurs at Wout = humidity ratio of air leaving [lb water/lb dry air] constant pressure (atmospheric for cooling towers), the heat In order to know how much heat the air flowing through a cooling absorbed in the air is directly correlated to the change in enthalpy. tower can absorb, the enthalpy of the air entering the tower must This is shown in Equation 1. be known. This is shown on the psychrometric chart Figure 1. G (hout - hin) = L x Cp (THW - 32°) - Cp (L - Le)(TCW - 32°) (1) The lines of constant enthalpy are close to parallel to the lines of constant wet bulb. -

Recommended Qualification Test Procedure for Solar Absorber.Pdf
Date: 2004-09-10 Editor Bo Carlsson1 IEA Solar Heating and Cooling Program Task 27 Performance of Solar Façade Components Project: Service life prediction tools for Solar Collectors Recommended qualification test procedure for solar absorber surface durability 1 Address: SP Swedish National Testing and Research Institute, P.O.Box 857, SE-50115 Borås, e-mail: [email protected] Contents Page Foreword.............................................................................................................................................................iv Introduktion .........................................................................................................................................................v 1 Scope ......................................................................................................................................................1 2 Normative references ............................................................................................................................1 3 Terms and definitions ...........................................................................................................................2 4 Requirements and classification..........................................................................................................3 5 Test methods for assessing material properties as measure of absorber performance...............4 5.1 Sampling and preparation of test specimens.....................................................................................4 -

A Comparative Energy and Economic Analysis Between a Low Enthalpy Geothermal Design and Gas, Diesel and Biomass Technologies for a HVAC System Installed in an Office Building
energies Article A Comparative Energy and Economic Analysis between a Low Enthalpy Geothermal Design and Gas, Diesel and Biomass Technologies for a HVAC System Installed in an Office Building José Ignacio Villarino 1, Alberto Villarino 1,* , I. de Arteaga 2 , Roberto Quinteros 2 and Alejandro Alañón 1 1 Department of Construction and Agronomy, Construction Engineering Area, High Polytechnic School of Ávila, University of Salamanca, Hornos Caleros, 50, 05003 Ávila, Spain; [email protected] (J.I.V.); [email protected] (A.A.) 2 Facultad de Ingeniería, Escuela de Ingeniería Mecánica, Pontificia Universidad Católica de Valparaíso, Av. Los Carrera 01567, Quilpué 2430000, Chile; [email protected] (I.d.A.); [email protected] (R.Q.) * Correspondence: [email protected]; Tel.: +34-920-353-500; Fax: +34-920-353-501 Received: 3 January 2019; Accepted: 25 February 2019; Published: 6 March 2019 Abstract: This paper presents an analysis of economic and energy between a ground-coupled heat pump system and other available technologies, such as natural gas, biomass, and diesel, providing heating, ventilation, and air conditioning to an office building. All the proposed systems are capable of reaching temperatures of 22 ◦C/25 ◦C in heating and cooling modes. EnergyPlus software was used to develop a simulation model and carry out the validation process. The first objective of the paper is the validation of the numerical model developed in EnergyPlus with the experimental results collected from the monitored building to evaluate the system in other operating conditions and to compare it with other available technologies. The second aim of the study is the assessment of the position of the low enthalpy geothermal system proposed versus the rest of the systems, from energy, economic, and environmental aspects. -

A Comprehensive Review of Thermal Energy Storage
sustainability Review A Comprehensive Review of Thermal Energy Storage Ioan Sarbu * ID and Calin Sebarchievici Department of Building Services Engineering, Polytechnic University of Timisoara, Piata Victoriei, No. 2A, 300006 Timisoara, Romania; [email protected] * Correspondence: [email protected]; Tel.: +40-256-403-991; Fax: +40-256-403-987 Received: 7 December 2017; Accepted: 10 January 2018; Published: 14 January 2018 Abstract: Thermal energy storage (TES) is a technology that stocks thermal energy by heating or cooling a storage medium so that the stored energy can be used at a later time for heating and cooling applications and power generation. TES systems are used particularly in buildings and in industrial processes. This paper is focused on TES technologies that provide a way of valorizing solar heat and reducing the energy demand of buildings. The principles of several energy storage methods and calculation of storage capacities are described. Sensible heat storage technologies, including water tank, underground, and packed-bed storage methods, are briefly reviewed. Additionally, latent-heat storage systems associated with phase-change materials for use in solar heating/cooling of buildings, solar water heating, heat-pump systems, and concentrating solar power plants as well as thermo-chemical storage are discussed. Finally, cool thermal energy storage is also briefly reviewed and outstanding information on the performance and costs of TES systems are included. Keywords: storage system; phase-change materials; chemical storage; cold storage; performance 1. Introduction Recent projections predict that the primary energy consumption will rise by 48% in 2040 [1]. On the other hand, the depletion of fossil resources in addition to their negative impact on the environment has accelerated the shift toward sustainable energy sources. -

Evaluation of the Effect of Relative Humidity of Air on the Coefficients of Critical Flow Venturi Nozzles
Evaluation of the Effect of Relative Humidity of Air on the Coefficients of Critical Flow Venturi Nozzles K. Chahine and M. Ballico National Measurement Institute, Australia P O Box 264, Lindfield, NSW 2070, Australia [email protected] Abstract atmospheric pressures. To establish stable sonic conditions in the nozzle, the down-stream end of the nozzle is usually connected to a high-capacity vacuum At NMIA, volumetric standards such as Brooks pump, with the up-stream end connected to the meter- or bell provers are used to calibrate critical flow Venturi under-test. During calibration the test-flowmeter draws nozzles or “sonic nozzles”. These nozzles, which are air from the laboratory at or near atmospheric pressures extremely stable, are used by both NMIA and Australian and temperature, and with relative humidity varying accredited laboratories to establish continuous flows for between 40% and 60%. The mass flowrate produced by the calibration of gas flow meters. For operational the nozzles is calculated based on the calibrated values of reasons, sonic nozzles are generally calibrated using dry the nozzle coefficient, the measured up-stream pressure air but later used with standard atmospheric air at various and the density calculated from the air temperature, humidity levels either drawn or blown through the meter- pressure and humidity [2]. under-test. Although the accepted theoretical calculations for determining the mass flow through a sonic nozzle At present, any effect of the relative humidity on the incorporate corrections for the resulting change in air nozzle coefficients is considered as negligible, as various density, as laboratories seek to reduce uncertainties the authors have estimated the systematic error at 0.02% for validity of this assumption warrants further examination. -

Psychrometrics Outline
Psychrometrics Outline • What is psychrometrics? • Psychrometrics in daily life and food industry • Psychrometric chart – Dry bulb temperature, wet bulb temperature, absolute humidity, relative humidity, specific volume, enthalpy – Dew point temperature • Mixing two streams of air • Heating of air and using it to dry a product 2 Psychrometrics • Psychrometrics is the study of properties of mixtures of air and water vapor • Water vapor – Superheated steam (unsaturated steam) at low pressure – Superheated steam tables are on page 817 of textbook – Properties of dry air are on page 818 of textbook – Psychrometric charts are on page 819 & 820 of textbook • What are these properties of interest and why do we need to know these properties? 3 Psychrometrics in Daily Life • Sea breeze and land breeze – When and why do we get them? • How do thunderstorms, hurricanes, and tornadoes form? • What are dew, fog, mist, and frost and when do they form? • When and why does the windshield of a car fog up? – How do you de-fog it? Is it better to blow hot air or cold air? Why? • Why do you feel dry in a heated room? – Is the moisture content of hot air lower than that of cold air? • How does a fan provide relief from sweating? • How does an air conditioner provide relief from sweating? • When does a soda can “sweat”? • When and why do we “see” our breath? • Do sailboats perform better at high or low relative humidity? Key factors: Temperature, Pressure, and Moisture Content of Air 4 Do Sailboats Perform Better at low or High RH? • Does dry air or moist air provide more thrust against the sail? • Which is denser – humid air or dry air? – Avogadro’s law: At the same temperature and pressure, the no. -

Cryogenicscryogenics Forfor Particleparticle Acceleratorsaccelerators Ph
CryogenicsCryogenics forfor particleparticle acceleratorsaccelerators Ph. Lebrun CAS Course in General Accelerator Physics Divonne-les-Bains, 23-27 February 2009 Contents • Low temperatures and liquefied gases • Cryogenics in accelerators • Properties of fluids • Heat transfer & thermal insulation • Cryogenic distribution & cooling schemes • Refrigeration & liquefaction Contents • Low temperatures and liquefied gases ••• CryogenicsCryogenicsCryogenics ininin acceleratorsacceleratorsaccelerators ••• PropertiesPropertiesProperties ofofof fluidsfluidsfluids ••• HeatHeatHeat transfertransfertransfer &&& thermalthermalthermal insulationinsulationinsulation ••• CryogenicCryogenicCryogenic distributiondistributiondistribution &&& coolingcoolingcooling schemesschemesschemes ••• RefrigerationRefrigerationRefrigeration &&& liquefactionliquefactionliquefaction • cryogenics, that branch of physics which deals with the production of very low temperatures and their effects on matter Oxford English Dictionary 2nd edition, Oxford University Press (1989) • cryogenics, the science and technology of temperatures below 120 K New International Dictionary of Refrigeration 3rd edition, IIF-IIR Paris (1975) Characteristic temperatures of cryogens Triple point Normal boiling Critical Cryogen [K] point [K] point [K] Methane 90.7 111.6 190.5 Oxygen 54.4 90.2 154.6 Argon 83.8 87.3 150.9 Nitrogen 63.1 77.3 126.2 Neon 24.6 27.1 44.4 Hydrogen 13.8 20.4 33.2 Helium 2.2 (*) 4.2 5.2 (*): λ Point Densification, liquefaction & separation of gases LNG Rocket fuels LIN & LOX 130 000 m3 LNG carrier with double hull Ariane 5 25 t LHY, 130 t LOX Air separation by cryogenic distillation Up to 4500 t/day LOX What is a low temperature? • The entropy of a thermodynamical system in a macrostate corresponding to a multiplicity W of microstates is S = kB ln W • Adding reversibly heat dQ to the system results in a change of its entropy dS with a proportionality factor T T = dQ/dS ⇒ high temperature: heating produces small entropy change ⇒ low temperature: heating produces large entropy change L. -

Matching Energy Consumption and Photovoltaic Production in a Retrofitted Dwelling in Subtropical Climate Without a Backup System
energies Article Matching Energy Consumption and Photovoltaic Production in a Retrofitted Dwelling in Subtropical Climate without a Backup System Sergio Gómez Melgar 1,* , Antonio Sánchez Cordero 2 , Marta Videras Rodríguez 2 and José Manuel Andújar Márquez 1 1 TEP192 Control y Robótica, Escuela Técnica Superior de Ingeniería, Universidad de Huelva, CP. 21007 Huelva, Spain; [email protected] 2 Programa de Ciencia y Tecnología Industrial y Ambiental, Escuela Técnica Superior de Ingeniería, Universidad de Huelva, CP. 21007 Huelva, Spain; [email protected] (A.S.C.); [email protected] (M.V.R.) * Correspondence: [email protected] Received: 4 October 2020; Accepted: 16 November 2020; Published: 18 November 2020 Abstract: The construction sector is a great contributor to global warming both in new and existing buildings. Minimum energy buildings (MEBs) demand as little energy as possible, with an optimized architectural design, which includes passive solutions. In addition, these buildings consume as low energy as possible introducing efficient facilities. Finally, they produce renewable energy on-site to become zero energy buildings (ZEBs) or even plus zero energy buildings (+ZEB). In this paper, a deep analysis of the energy use and renewable energy production of a social dwelling was carried out based on data measurements. Unfortunately, in residential buildings, most renewable energy production occurs at a different time than energy demand. Furthermore, energy storage batteries for these facilities are expensive and require significant maintenance. The present research proposes a strategy, which involves rescheduling energy demand by changing the habits of the occupants in terms of domestic hot water (DHW) consumption, cooking, and washing. -

Installation, Setup & Troubleshooting Supplement
EconoMi$er X F a c t o r y --- I n s t a l l e d O p t i o n Low Leak Economizer for 2 Speed SAV (Staged Air Volume) Systems Installation, Setup & Troubleshooting Supplement This document is a supplemental installation instruction for the factory-installed EconoMi$er X (low leak economizer) option. It is to be used with the base unit Installation Instructions for 48/50TC, 50TCQ, 48/50HC, and 50HCQ 2-Stage cooling units, sizes 08 – 30. Units equipped with the EconoMi$er X option are identified by an indicator in the unit's model number (see the unit's nameplate). Use Table 1 (on page 2) to identify whether or not a given unit is equipped with the factory-installed EconoMi$er X. NOTE: Read the entire instruction manual before starting the installation. TABLE OF CONTENTS SAFETY CONSIDERATIONS SAFETY CONSIDERATIONS.................... 1 Improper installation, adjustment, alteration, service, maintenance, or use can cause explosion, fire, electrical GENERAL.................................... 2 shock or other conditions which may cause personal injury Identifying Factory Option...................... 2 or property damage. Consult a qualified installer, service EconoMi$er X................................ 3 agency, or your distributor or branch for information or assistance. The qualified installer or agency must use W7220 Economizer Controller................... 3 factory--authorized kits or accessories when modifying this User Interface................................ 3 product. Refer to the individual instructions packaged with Menu Structure............................... 3 the kits or accessories when installing. Checkout Tests............................... 7 Follow all safety codes. Wear safety glasses and work gloves. Use quenching cloths for brazing operations and SETUP AND CONFIGURATION................