Why Are Some Lakes Salty?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Altai Region, Russia)
Limnology and Freshwater Biology 2020 (4): 1019-1020 DOI:10.31951/2658-3518-2020-A-4-1019 SI: “The V-th Baikal Symposium on Microbiology” Short communication Salt-dependent succession of phototrophic communities in the soda lake Tanatar VI (Altai Region, Russia) Samylina O.S.1*, Namsaraev Z.B.2 1 Winogradsky Institute of Microbiology, Research Center of Biotechnology, Russian Academy of Sciences, 60 let Oktjabrja pr-t, 7-2, Moscow, 117312, Russia 2 NRC “Kurchatov Institute”, Akademika Kurchatova pl., 1, Moscow, 123182, Russia ABSTRACT. The paper describes the changes that occurred in the ecosystem of soda lake Tanatar VI during the 2011-2019 period when salinity decreased from 160-250 to 13-14 g/l Keywords: soda lake, Picocystis, cyanobacteria, biological soil crusts, succession, nitrogen fixation 1. Introduction salinity. Several types of phototrophic communities were detected in the Tanatar VI (Table): 1) Bottom, A great number and variety of saline lakes are floating and epiphytic cyanobacterial biofilms (CB); located in the Kulunda Steppe in the Altai Region. 2) floating and epiphytic Ctenocladus-communities These lakes are exposed to annual and long-standing with filamentous chlorophyte Ctenocladus circinnatus fluctuations of temperature, salinity, flooding and and cyanobacteria (CC); 3) Blooms of unicellular drying periods due to their locality in the zone of algae Picocystis salinarum (P-bloom); 4) Cyanobacterial cold arid climate. There are soda lakes among them, blooms (CB-bloom); 5) Biological soil crusts (BSC) which maintain stable alkaline pH due to a prevalence developed on the moist soil between thickets of of soluble carbonates in the brines. Thereby diversity Salicornia altaica near the lake. -

Historical Volcanism and the State of Stress in the East African Rift System
Historical volcanism and the state of stress in the East African Rift System Article Accepted Version Open Access Wadge, G., Biggs, J., Lloyd, R. and Kendall, J.-M. (2016) Historical volcanism and the state of stress in the East African Rift System. Frontiers in Earth Science, 4. 86. ISSN 2296- 6463 doi: https://doi.org/10.3389/feart.2016.00086 Available at http://centaur.reading.ac.uk/66786/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . To link to this article DOI: http://dx.doi.org/10.3389/feart.2016.00086 Publisher: Frontiers media All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online 1 Historical volcanism and the state of stress in the East African 2 Rift System 3 4 5 G. Wadge1*, J. Biggs2, R. Lloyd2, J-M. Kendall2 6 7 8 1.COMET, Department of Meteorology, University of Reading, Reading, UK 9 2.COMET, School of Earth Sciences, University of Bristol, Bristol, UK 10 11 * [email protected] 12 13 14 Keywords: crustal stress, historical eruptions, East African Rift, oblique motion, 15 eruption dynamics 16 17 18 19 20 21 Abstract 22 23 Crustal extension at the East African Rift System (EARS) should, as a tectonic ideal, 24 involve a stress field in which the direction of minimum horizontal stress is 25 perpendicular to the rift. -

When “Evaporites” Are Not Formed by Evaporation: the Role Of
When “evaporites” are not formed by evaporation: The role of temperature and pCO2 on saline deposits of the Eocene Green River Formation, Colorado, USA Robert V. Demicco† and Tim K. Lowenstein Department of Geological Sciences and Environmental Studies, Binghamton University, Binghamton, New York 13902-6000, USA ABSTRACT INTRODUCTION rated with halite. During summer, the waters of the lake above the thermocline (at <25 m depth) Halite precipitates in the Dead Sea during Geologists studying salt deposits have noticed warm to ∼34 °C, become undersaturated with ha- winter but re-dissolves above the thermo- that very soluble minerals that occur in the cen- lite due to the temperature increase, and the bulk cline upon summer warming, “focusing” ha- ter of a basin do not extend out to the edges of of the winter-deposited halite above the thermo- lite deposition below the thermocline (Sirota the basin (Hsü et al., 1973; Dyni, 1981; Lowen- cline re-dissolves. Summer dissolution of halite et al., 2016, 2017, 2018). Here we develop an stein, 1988). This is true even where: (1) soluble occurs in the Dead Sea despite any increase in “evaporite focusing” model for evaporites salts are interpreted to have been deposited in evaporative concentration at the surface. The ha- (nahcolite + halite) preserved in a restricted a deep evaporitic lake or marine basin and (2) lite that settled into the deeper, cooler isothermal area of the Eocene Green River Formation deep-water deposits that encase salts in the cen- bottom waters, however, accumulates as an an- in the Piceance Creek Basin of Colorado, ter of the basin can be confidently traced to pe- nual layer at depths below the 25 m deep ther- USA. -

Metagenomic Insights Into the Uncultured Diversity and Physiology of Microbes in Four Hypersaline Soda Lake Brines
Lawrence Berkeley National Laboratory Recent Work Title Metagenomic Insights into the Uncultured Diversity and Physiology of Microbes in Four Hypersaline Soda Lake Brines. Permalink https://escholarship.org/uc/item/9xc5s0v5 Journal Frontiers in microbiology, 7(FEB) ISSN 1664-302X Authors Vavourakis, Charlotte D Ghai, Rohit Rodriguez-Valera, Francisco et al. Publication Date 2016 DOI 10.3389/fmicb.2016.00211 Peer reviewed eScholarship.org Powered by the California Digital Library University of California ORIGINAL RESEARCH published: 25 February 2016 doi: 10.3389/fmicb.2016.00211 Metagenomic Insights into the Uncultured Diversity and Physiology of Microbes in Four Hypersaline Soda Lake Brines Charlotte D. Vavourakis 1, Rohit Ghai 2, 3, Francisco Rodriguez-Valera 2, Dimitry Y. Sorokin 4, 5, Susannah G. Tringe 6, Philip Hugenholtz 7 and Gerard Muyzer 1* 1 Microbial Systems Ecology, Department of Aquatic Microbiology, Institute for Biodiversity and Ecosystem Dynamics, University of Amsterdam, Amsterdam, Netherlands, 2 Evolutionary Genomics Group, Departamento de Producción Vegetal y Microbiología, Universidad Miguel Hernández, San Juan de Alicante, Spain, 3 Department of Aquatic Microbial Ecology, Biology Centre of the Czech Academy of Sciences, Institute of Hydrobiology, Ceskéˇ Budejovice,ˇ Czech Republic, 4 Research Centre of Biotechnology, Winogradsky Institute of Microbiology, Russian Academy of Sciences, Moscow, Russia, 5 Department of Biotechnology, Delft University of Technology, Delft, Netherlands, 6 The Department of Energy Joint Genome Institute, Walnut Creek, CA, USA, 7 Australian Centre for Ecogenomics, School of Chemistry and Molecular Biosciences and Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD, Australia Soda lakes are salt lakes with a naturally alkaline pH due to evaporative concentration Edited by: of sodium carbonates in the absence of major divalent cations. -

Alcolapia Grahami ERSS
Lake Magadi Tilapia (Alcolapia grahami) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, March 2015 Revised, August 2017, October 2017 Web Version, 8/21/2018 1 Native Range and Status in the United States Native Range From Bayona and Akinyi (2006): “The natural range of this species is restricted to a single location: Lake Magadi [Kenya].” Status in the United States No records of Alcolapia grahami in the wild or in trade in the United States were found. The Florida Fish and Wildlife Conservation Commission has listed the tilapia Alcolapia grahami as a prohibited species. Prohibited nonnative species (FFWCC 2018), “are considered to be dangerous to the ecology and/or the health and welfare of the people of Florida. These species are not allowed to be personally possessed or used for commercial activities.” Means of Introductions in the United States No records of Alcolapia grahami in the United States were found. 1 Remarks From Bayona and Akinyi (2006): “Vulnerable D2 ver 3.1” Various sources use Alcolapia grahami (Eschmeyer et al. 2017) or Oreochromis grahami (ITIS 2017) as the accepted name for this species. Information searches were conducted under both names to ensure completeness of the data gathered. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing According to Eschmeyer et al. (2017), Alcolapia grahami (Boulenger 1912) is the current valid name for this species. It was originally described as Tilapia grahami; it has also been known as Oreoghromis grahami, and as a synonym, but valid subspecies, of -

Hidden Diversity of Plankton in the Soda Lake Nakuru, Kenya, During A
Hydrobiologia (2013) 702:95–103 DOI 10.1007/s10750-012-1310-y PRIMARY RESEARCH PAPER Hidden diversity of eukaryotic plankton in the soda lake Nakuru, Kenya, during a phase of low salinity revealed by a SSU rRNA gene clone library Wei Luo • Kiplagat Kotut • Lothar Krienitz Received: 12 July 2012 / Revised: 28 August 2012 / Accepted: 31 August 2012 / Published online: 16 September 2012 Ó Springer Science+Business Media B.V. 2012 Abstract A SSU rRNA gene clone library was Our findings reveal a hidden diversity, which would constructed to establish the diversity of eukaryotic not have been detected by traditional observations. plankton in the African soda lake Nakuru during a phase of low salinity (9.7 ppt = hyposaline). Nor- Keywords Chlorophyta Á Clone library Á Lake mally, the lake is mesosaline (up to 50 ppt) and its Nakuru Á Lesser Flamingo Á Phytoflagellates Á phytoplankton is dominated by few species of cyano- Soda lakes Á SSU rRNA bacteria, in particular Arthrospira fusiformis, which is the main food resource of Lesser Flamingos. Our study recovered a unique phytoplankton species composi- Introduction tion characterized by a high diversity of monadoid and coccoid green algae. Out of 77 clones detected, 52 The phytoplankton of saline-alkaline lakes in the Great belonged to Chlorophyta. Many of the chlorophytes African Rift Valley usually is dominated by cyano- were transported from the catchment area into the lake bacteria, mainly Arthrospira fusiformis (Voronichin) through small seasonal rivers and an outflow of the Koma´rek et Lund (often erroneously identified as Nakuru town sewage treatment plant. Other phyloge- ‘‘Spirulina platensis’’), which forms the food base for netic groups detected were Fungi, Cryptophyta, hundreds of thousands of Lesser Flamingos (Phoenic- Jakobida, Alveolata, Stramenopiles, and Metazoa. -

SODIUM BICARBONATE (NAHCOLITE) from COLORADOOIL SHALE1 Tbr-R-Enrr-2
SODIUM BICARBONATE (NAHCOLITE) FROM COLORADOOIL SHALE1 TBr-r-Enrr-2 Assrnact Sodium bicarbonate (nahcolite) was found in the high-grade oil-shale zone of tfre Para- chute Creek member of the Eocene Green River formation in ttre underground development openings of ttre Bureau of Mines Oil-Shale Demonstration Mine at Anvil Points, 10 miles west of Rifle, Colo. It occurs as concretions varying up to five feet in diameter and as layers up to four inches thick, intercalated between rich oil-shale beds. It is suggested tlat the concretions were formed while the ooze, which had been deposited in the bottom of the former Uinta Lake, was still soft and plastic. INrnorucrrorq Natural sodium bicarbonate (NaHCOa) was reported first by P. Wal- thera from Little Magadi dry lake, in British East Africa, but no con- flmatory evidence was given. F. A. Bannister,a who reported natural sodium bicarbonate in an efforescencefrom an old Roman underground conduit from hot springs at Stufe de Nerone, near Naples, gave the min- eral the name "nahcolite." Later the mineral was reported by E. Quer- cighs as an incrustation in a lava grotto, apparently mixed with thenar- dite and halite. Large quantities of nahcolite were found in a well core- dritled below the central salt crust of Searles Lake and described by William F. Foshag.6 The presenceof saline phasesin the eastern part of the Uinta Basin in the high-grade oil-shale facies of the Parachute Creek member of the Eocene Green Eiver formation was noted by W. H. Bradley.T He states that ellipsoidal cavities whose major axes range from a fraction of an inch to more than five feet in length contained molds of radial aggregates of a saline mineral that could not be determined becauseof the complex intergrowth of the crystal molds. -

24-13 Robert
www.roavs.com EISSN: 2223-0343 Research Opinions in Animal & Veterinary Sciences Cyanobacterial toxins and bacterial infections are the possible causes of mass mortality of lesser flamingos in Soda lakes in northern Tanzania Robert D. Fyumagwa 1* , Zablon Bugwesa 1, Machoke Mwita 1, Emilian S. Kihwele 2, Athanas Nyaki 3, Robinson H. Mdegela 4 and Donald G. Mpanduji 4 1Tanzania Wildlife Research Institute (TAWIRI), P.O.Box 661, Arusha, Tanzania; 2Serengeti Nattional Park P. O. Box 3134, Arusha, Tanzania; 3Ngorongoro Conservation Area Authority (NCAA) P. O. Box 1, Ngorongoro, Aursha, Tanzania; 4Faculty of Veterinary Medicine, Sokoine University of Agriculture (SUA), P. O. Box 3022, Morogoro, Tanzania Abstract During the mass die-off of lesser flamingos in Soda lakes of Tanzania in 2000, 2002 and 2004, clinicopathological and toxicological investigations were made in order to elucidate the likely cause of mortality. Water and tissue samples were collected from the lakes and from dead flamingos respectively. While water samples were analyzed for pesticide residues, tissues were analyzed for pesticide residues and cyanotoxins. The significant pathological lesions observed in fresh carcasses included oedema in lungs, enlarged liver, haemorrhages in liver with multiple necrotic foci, haemorrhages in kidneys and haemorrhages in intestines with erosion of mucosa. Analysis of cyanotoxins revealed presence of neurotoxin (anatoxin-a) and hepatotoxins (microcystins LR, RR). Concentrations of microcystins LR were significantly higher (P = 0.0003) in liver than in other tissues. Based on clinicopathological findings and concentrations of the detected cyanotoxins, it is suspected that cyanobacterial toxins concurrent with secondary bacterial infection were the likely cause of the observed mortalities in flamingos. -

The Soda Lakes of Nhecolândia: a Conservation Opportunity for The
Perspectives in Ecology and Conservation 17 (2019) 9–18 ´ Supported by Boticario Group Foundation for Nature Protection www.perspectecolconserv.com Essays and Perspectives The soda lakes of Nhecolândia: A conservation opportunity for the Pantanal wetlands a b c,∗ d Renato L. Guerreiro , Ivan Bergier , Michael M. McGlue , Lucas V. Warren , b e d Urbano Gomes Pinto de Abreu , Jônatas Abrahão , Mario L. Assine a Instituto Federal de Educac¸ ão, Ciência e Tecnologia do Paraná, Av. Cívica, 475, 85935-000 Assis Chateaubriand, PR, Brazil b Embrapa Pantanal, Rua 21 de Setembro, 1880, 79302-090 Corumbá, MS, Brazil c Department of Earth and Environmental Sciences, University of Kentucky, 121 Washington Ave, Lexington, KY 40506, USA d Instituto de Geociências e Ciências Exatas, Unesp – Universidade Estadual Paulista, Avenida 24-A, Bela Vista, Rio Claro, SP CEP 13506-900, Brazil e Laboratório de Vírus, Instituto de Ciências Biológicas, Departamento de Microbiologia, Universidade Federal de Minas Gerais, Belo Horizonte 31270-901, Brazil a a b s t r a c t r t i c l e i n f o Article history: The Pantanal is the most conserved biome in Brazil and among the last wild refuges in South Amer- Received 2 July 2018 ica, but intensification of agriculture and other land use changes present challenges for protecting this Accepted 26 November 2018 exceptionally biodiverse wetland ecosystem. Recent studies have shed new light on the origins and bio- Available online 11 December 2018 geochemistry of a suite of >600 small saline-alkaline lakes in Nhecolândia, a floodplain setting located south of the Taquari River in south-central Pantanal. -
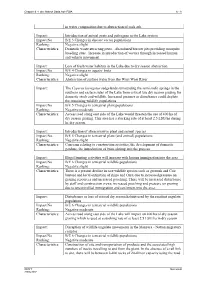
In Water Composition Due to Abstraction of Soda Ash. Impact
Chapter 6 - Lake Natron Soda Ash ESIA 6 - 8 in water composition due to abstraction of soda ash. Impact: Introduction of animal pests and pathogens to the Lake system Impact No. B/E 3 Changes in disease vector populations Ranking: Negative slight Characteristics: Domestic waste attracting pests. Abandoned borrow pits providing mosquito breeding sites. Increase in introduction of vectors through increased human and vehicle movement. Impact: Loss of fresh water habitats in the Lake due to dry season abstraction Impact No. B/E 4 Changes in aquatic biota Ranking: Negative slight Characteristics: Abstraction of surface water from the Wosi Wosi River Impact: The Cyperus laevigatus sedgelands surrounding the semi sodic springs in the southern and eastern sides of the Lake form critical late dry season grazing for domestic stock and wildlife. Increased pressure or disturbance could deplete the remaining wildlife populations Impact No. B/E 5 Changes in terrestrial plant populations Ranking: Negative moderate Characteristics: Access road along east side of the Lake would threaten the use of 400 ha of dry season grazing. This area has a stocking rate of at least 2.5 LSU/ha during he dry season Impact: Introduction of alien invasive plant and animal species Impact No. B/E 5 Changes in terrestrial plant (and animal) populations Ranking: Negative slight Characteristics: Concerns relating to construction activities, the development of domestic gardens, the introduction of brine shrimp into the process Impact: Illegal hunting activities will increase with human immigration into the area Impact No. B/E 6 Changes in terrestrial wildlife populations Ranking: Negative slight Characteristics: There is a present decline in rare wildlife species such as gerenuk and Coir bustard and local extinction of rhino and Oryx due to increased pressure on grazing resources and increased poaching. -

Geology Area South of Magadi
_£I Report No. 61 GOVERNMENT OF KENYA MINISTRY OF COMMERCE AND INDUSTRY GEOLOGICAL SURVEY OF KENYA GEOLOGY OF THE AREA SOUTH OF MAGADI DEGREE SHEET 58, N.W. QUARTER (with coloured geological map) by B. H. BAKER, B.Sc, F.G.S. Geologist Eight Shillings - 1963 "ISfiICrLIBSARY ïIE-- :i963l4 j». ^itfageningen _ .The'Netherlands Li / J Scanned from original by ISRIC - World Soil Information, as ICSU World Data Centre for Soils. The purpose is to make a safe depository for endangered documents and to make the accrued information available for consultation, following Fair Use Guidelines. Every effort is taken to respect Copyright of the materials within the archives where the identification of the Copyright holder is clear and, where feasible, to contact the originators. For questions please contact soil.isricPwur.nl indicating the item reference number concerned. GEOLOGY OF THE AREA SOUTH OF MAGADI DEGREE SHEET 58, N.W. QUARTER (with coloured geological map) by B. H. BAKER, B.Sc, F.G.S. Geologist 165^G FOREWORD The publication of the report on the geology of the area south of Magadi completes the account of the southern end of the Rift Valley as it occurs in Kenya. The Magadi area itself was described by Mr. Baker in Report No. 42 (1958). During the mapping of the continua tion of the Magadi area the discovery of some critical exposures enabled the correction of an error of succession in the lower Pleistocene rocks that had been made during the survey of the Magadi area. The area is wild and desolate, but of considerable interest scenically, with the western Rift wall a little beyond its west boundary, rugged hills of ancient rocks in the south-east and two prominent volcanoes, Lenderut and Shombole, rising from the Rift floor. -

Rift-Valley-1.Pdf
R E S O U R C E L I B R A R Y E N C Y C L O P E D I C E N T RY Rift Valley A rift valley is a lowland region that forms where Earth’s tectonic plates move apart, or rift. G R A D E S 6 - 12+ S U B J E C T S Earth Science, Geology, Geography, Physical Geography C O N T E N T S 9 Images For the complete encyclopedic entry with media resources, visit: http://www.nationalgeographic.org/encyclopedia/rift-valley/ A rift valley is a lowland region that forms where Earth’s tectonic plates move apart, or rift. Rift valleys are found both on land and at the bottom of the ocean, where they are created by the process of seafloor spreading. Rift valleys differ from river valleys and glacial valleys in that they are created by tectonic activity and not the process of erosion. Tectonic plates are huge, rocky slabs of Earth's lithosphere—its crust and upper mantle. Tectonic plates are constantly in motion—shifting against each other in fault zones, falling beneath one another in a process called subduction, crashing against one another at convergent plate boundaries, and tearing apart from each other at divergent plate boundaries. Many rift valleys are part of “triple junctions,” a type of divergent boundary where three tectonic plates meet at about 120° angles. Two arms of the triple junction can split to form an entire ocean. The third, “failed rift” or aulacogen, may become a rift valley.