Phylogeny of the Tribe Antirrhineae (Scrophulariaceae) Based on Morphological and Ndhf Sequence Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Central Appalachian Broadleaf Forest Coniferous Forest Meadow Province
Selecting Plants for Pollinators A Regional Guide for Farmers, Land Managers, and Gardeners In the Central Appalachian Broadleaf Forest Coniferous Forest Meadow Province Including the states of: Maryland, Pennsylvania, Virginia, West Virginia And parts of: Georgia, Kentucky, and North Carolina, NAPPC South Carolina, Tennessee Table of CONTENTS Why Support Pollinators? 4 Getting Started 5 Central Appalachian Broadleaf Forest 6 Meet the Pollinators 8 Plant Traits 10 Developing Plantings 12 Far ms 13 Public Lands 14 Home Landscapes 15 Bloom Periods 16 Plants That Attract Pollinators 18 Habitat Hints 20 This is one of several guides for Check list 22 different regions in the United States. We welcome your feedback to assist us in making the future Resources and Feedback 23 guides useful. Please contact us at [email protected] Cover: silver spotted skipper courtesy www.dangphoto.net 2 Selecting Plants for Pollinators Selecting Plants for Pollinators A Regional Guide for Farmers, Land Managers, and Gardeners In the Ecological Region of the Central Appalachian Broadleaf Forest Coniferous Forest Meadow Province Including the states of: Maryland, Pennsylvania, Virginia, West Virginia And parts of: Georgia, Kentucky, North Carolina, South Carolina, Tennessee a nappc and Pollinator Partnership™ Publication This guide was funded by the National Fish and Wildlife Foundation, the C.S. Fund, the Plant Conservation Alliance, the U.S. Forest Service, and the Bureau of Land Management with oversight by the Pollinator Partnership™ (www.pollinator.org), in support of the North American Pollinator Protection Campaign (NAPPC–www.nappc.org). Central Appalachian Broadleaf Forest – Coniferous Forest – Meadow Province 3 Why support pollinators? In theIr 1996 book, the Forgotten PollInators, Buchmann and Nabhan estimated that animal pollinators are needed for the reproduction “ Farming feeds of 90% of flowering plants and one third of human food crops. -

The Plant Press the ARIZONA NATIVE PLANT SOCIETY
The Plant Press THE ARIZONA NATIVE PLANT SOCIETY Volume 36, Number 1 Summer 2013 In this Issue: Plants of the Madrean Archipelago 1-4 Floras in the Madrean Archipelago Conference 5-8 Abstracts of Botanical Papers Presented in the Madrean Archipelago Conference Southwest Coralbean (Erythrina flabelliformis). Plus 11-19 Conservation Priority Floras in the Madrean Archipelago Setting for Arizona G1 Conference and G2 Plant Species: A Regional Assessment by Thomas R. Van Devender1. Photos courtesy the author. & Our Regular Features Today the term ‘bioblitz’ is popular, meaning an intensive effort in a short period to document the diversity of animals and plants in an area. The first bioblitz in the southwestern 2 President’s Note United States was the 1848-1855 survey of the new boundary between the United States and Mexico after the Treaty of Guadalupe Hidalgo of 1848 ended the Mexican-American War. 8 Who’s Who at AZNPS The border between El Paso, Texas and the Colorado River in Arizona was surveyed in 1855- 9 & 17 Book Reviews 1856, following the Gadsden Purchase in 1853. Besides surveying and marking the border with monuments, these were expeditions that made extensive animal and plant collections, 10 Spotlight on a Native often by U.S. Army physicians. Botanists John M. Bigelow (Charphochaete bigelovii), Charles Plant C. Parry (Agave parryi), Arthur C. V. Schott (Stephanomeria schotti), Edmund K. Smith (Rhamnus smithii), George Thurber (Stenocereus thurberi), and Charles Wright (Cheilanthes wrightii) made the first systematic plant collection in the Arizona-Sonora borderlands. ©2013 Arizona Native Plant In 1892-94, Edgar A. Mearns collected 30,000 animal and plant specimens on the second Society. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Conserving Europe's Threatened Plants
Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation By Suzanne Sharrock and Meirion Jones May 2009 Recommended citation: Sharrock, S. and Jones, M., 2009. Conserving Europe’s threatened plants: Progress towards Target 8 of the Global Strategy for Plant Conservation Botanic Gardens Conservation International, Richmond, UK ISBN 978-1-905164-30-1 Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, Surrey, TW9 3BW, UK Design: John Morgan, [email protected] Acknowledgements The work of establishing a consolidated list of threatened Photo credits European plants was first initiated by Hugh Synge who developed the original database on which this report is based. All images are credited to BGCI with the exceptions of: We are most grateful to Hugh for providing this database to page 5, Nikos Krigas; page 8. Christophe Libert; page 10, BGCI and advising on further development of the list. The Pawel Kos; page 12 (upper), Nikos Krigas; page 14: James exacting task of inputting data from national Red Lists was Hitchmough; page 16 (lower), Jože Bavcon; page 17 (upper), carried out by Chris Cockel and without his dedicated work, the Nkos Krigas; page 20 (upper), Anca Sarbu; page 21, Nikos list would not have been completed. Thank you for your efforts Krigas; page 22 (upper) Simon Williams; page 22 (lower), RBG Chris. We are grateful to all the members of the European Kew; page 23 (upper), Jo Packet; page 23 (lower), Sandrine Botanic Gardens Consortium and other colleagues from Europe Godefroid; page 24 (upper) Jože Bavcon; page 24 (lower), Frank who provided essential advice, guidance and supplementary Scumacher; page 25 (upper) Michael Burkart; page 25, (lower) information on the species included in the database. -

January 2012 ---International Rock Gardener--- January 2012
International Rock Gardener Number 25 The Scottish Rock Garden Club January 2012 ---International Rock Gardener--- January 2012 We begin the year with some “perennial favourites”: plants with lasting attraction. The late Harold Esslemont was one of the most experienced growers and exhibitors in the SRGC over a great many years and the following article was adapted from The Rock Garden journal of 1969 to showcase some plants that are as popular today as they were over forty years ago. The last cover of 2011 was of a wintry scene in the Scottish Garden of two of the IRG team so we thought we’d share this January sunset for the start of 2012. In his weekly Bulb Log Diary, now in its tenth year, Ian shares his method of taking such photos. Cover picture: January Sunset, Aberdeen. J. Ian Young ---Mountains in the Garden--- My Twelve Favourite Alpines by the late Harold Esslemont M.B.E. (adapted by M.Y.) It was the final meeting of the season of the local group. A postcard announced that two* members had been invited to show and discuss slides of their twelve favourite alpines. It appeared that I was to be one of the speakers. I forget who told me that his list of twelve favourite alpines ran to at least twenty, but I was soon to learn how right he was. My brief was twelve plants, no more, and a decision, however difficult, had to be made. The compiling of such a list is influenced by so many factors that the result may be expected to vary widely among individuals. -

Different Speciation Types Meet in a Mediterranean Genus: The
This is an Accepted Manuscript of an article published in Taxon on 4 May 2017, available online: https://doi.org/10.12705/662.7 Different speciation types meet in a Mediterranean genus: the biogeographic history of Cymbalaria (Plantaginaceae). Running head: Phylogeny and biogeographic history of Cymbalaria Pau Carnicero1, Llorenç Sáez1, 2, Núria Garcia-Jacas3 and Mercè Galbany- Casals1 1 Departament de Biologia Animal, Biologia Vegetal i Ecologia, Facultat de Biociències, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain. 2 Societat d’Història Natural de les Balears (SHNB), C/ Margarida Xirgu 16, E-07011 Palma de Mallorca, Balearic Islands, Spain. 3 Institut Botànic de Barcelona (IBB-CSIC-ICUB), Pg. del Migdia s/n, ES-08038 Barcelona, Spain Author for correspondence: Pau Carnicero, [email protected] ORCID: P.C., http://orcid.org/0000-0002-8345-3309 Abstract Cymbalaria comprises ten species and six subspecies growing in rocky habitats in the Mediterranean Basin. Several features, such as the genus’ highly fragmented distribution as well as noticeable ecological differentiation between partially sympatric species and presence of ploidy barriers between species suggest the involvement of different speciation types in its evolution. The aims of this study were to test the monophyly of Cymbalaria and to reconstruct infrageneric phylogenetic relationships, to infer the genus’ biogeographic history by estimating divergence times and ancestral distribution areas of lineages, and to disentangle the role of different speciation types. To address these issues, we constructed a phylogeny with a complete taxon sampling based on ITS, 3'ETS, ndhF and rpl32-trnL sequences. We used the nuclear ribosomal DNA data to produce a time-calibrated phylogeny, which served as basis for estimating ploidy level evolution and biogeographic history. -

Host Plants and Habitats of the Baltimore Checkerspot Butterfly, Euphydryas Phaeton (Lepidoptera: Nymphalidae), in the Great Lakes Region
The Great Lakes Entomologist Volume 24 Number 4 - Winter 1991 Number 4 - Winter Article 1 1991 December 1991 Host Plants and Habitats of the Baltimore Checkerspot Butterfly, Euphydryas Phaeton (Lepidoptera: Nymphalidae), in the Great Lakes Region Brian G. Scholtens University of Michigan Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Scholtens, Brian G. 1991. "Host Plants and Habitats of the Baltimore Checkerspot Butterfly, Euphydryas Phaeton (Lepidoptera: Nymphalidae), in the Great Lakes Region," The Great Lakes Entomologist, vol 24 (4) Available at: https://scholar.valpo.edu/tgle/vol24/iss4/1 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Scholtens: Host Plants and Habitats of the Baltimore Checkerspot Butterfly, 1991 THE GREAT LAKES ENTOMOLOGIST 207 HOST PLANTS AND HABITATS OF THE BALTIMORE CHECKERSPOT BUTTERFLY, EUPHYDRYAS PHAETON (LEPIDOPTERA: NYMPHALIDAE), IN THE GREAT LAKES REGION Brian G. Scholtens 1 ABSTRACT The habitats and host plants of Euphydryas phaeton in the Great Lakes region are examined using data from several different populations spread over much of the region. The range of habitats and host plants used by this species is wider than commonly believed. While many populations are found in seasonal or permanent wetlands, others are located in dry, old fields or woodland areas. The host plants used vary with habitat, but they include all major primary hosts and many second ary hosts previously reported plus several new records. -

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -

Pollen and Stamen Mimicry: the Alpine Flora As a Case Study
Arthropod-Plant Interactions DOI 10.1007/s11829-017-9525-5 ORIGINAL PAPER Pollen and stamen mimicry: the alpine flora as a case study 1 1 1 1 Klaus Lunau • Sabine Konzmann • Lena Winter • Vanessa Kamphausen • Zong-Xin Ren2 Received: 1 June 2016 / Accepted: 6 April 2017 Ó The Author(s) 2017. This article is an open access publication Abstract Many melittophilous flowers display yellow and Dichogamous and diclinous species display pollen- and UV-absorbing floral guides that resemble the most com- stamen-imitating structures more often than non-dichoga- mon colour of pollen and anthers. The yellow coloured mous and non-diclinous species, respectively. The visual anthers and pollen and the similarly coloured flower guides similarity between the androecium and other floral organs are described as key features of a pollen and stamen is attributed to mimicry, i.e. deception caused by the flower mimicry system. In this study, we investigated the entire visitor’s inability to discriminate between model and angiosperm flora of the Alps with regard to visually dis- mimic, sensory exploitation, and signal standardisation played pollen and floral guides. All species were checked among floral morphs, flowering phases, and co-flowering for the presence of pollen- and stamen-imitating structures species. We critically discuss deviant pollen and stamen using colour photographs. Most flowering plants of the mimicry concepts and evaluate the frequent evolution of Alps display yellow pollen and at least 28% of the species pollen-imitating structures in view of the conflicting use of display pollen- or stamen-imitating structures. The most pollen for pollination in flowering plants and provision of frequent types of pollen and stamen imitations were pollen for offspring in bees. -

Alien Flora of Europe: Species Diversity, Temporal Trends, Geographical Patterns and Research Needs
Preslia 80: 101–149, 2008 101 Alien flora of Europe: species diversity, temporal trends, geographical patterns and research needs Zavlečená flóra Evropy: druhová diverzita, časové trendy, zákonitosti geografického rozšíření a oblasti budoucího výzkumu Philip W. L a m b d o n1,2#, Petr P y š e k3,4*, Corina B a s n o u5, Martin H e j d a3,4, Margari- taArianoutsou6, Franz E s s l7, Vojtěch J a r o š í k4,3, Jan P e r g l3, Marten W i n t e r8, Paulina A n a s t a s i u9, Pavlos A n d r i opoulos6, Ioannis B a z o s6, Giuseppe Brundu10, Laura C e l e s t i - G r a p o w11, Philippe C h a s s o t12, Pinelopi D e l i p e t - rou13, Melanie J o s e f s s o n14, Salit K a r k15, Stefan K l o t z8, Yannis K o k k o r i s6, Ingolf K ü h n8, Hélia M a r c h a n t e16, Irena P e r g l o v á3, Joan P i n o5, Montserrat Vilà17, Andreas Z i k o s6, David R o y1 & Philip E. H u l m e18 1Centre for Ecology and Hydrology, Hill of Brathens, Banchory, Aberdeenshire AB31 4BW, Scotland, e-mail; [email protected], [email protected]; 2Kew Herbarium, Royal Botanic Gardens Kew, Richmond, Surrey, TW9 3AB, United Kingdom; 3Institute of Bot- any, Academy of Sciences of the Czech Republic, CZ-252 43 Průhonice, Czech Republic, e-mail: [email protected], [email protected], [email protected], [email protected]; 4Department of Ecology, Faculty of Science, Charles University, Viničná 7, CZ-128 01 Praha 2, Czech Republic; e-mail: [email protected]; 5Center for Ecological Research and Forestry Applications, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain, e-mail: [email protected], [email protected]; 6University of Athens, Faculty of Biology, Department of Ecology & Systematics, 15784 Athens, Greece, e-mail: [email protected], [email protected], [email protected], [email protected], [email protected]; 7Federal Environment Agency, Department of Nature Conservation, Spittelauer Lände 5, 1090 Vienna, Austria, e-mail: [email protected]; 8Helmholtz Centre for Environmental Research – UFZ, Department of Community Ecology, Theodor-Lieser- Str. -

Introduction to the Southern Blue Ridge Ecoregional Conservation Plan
SOUTHERN BLUE RIDGE ECOREGIONAL CONSERVATION PLAN Summary and Implementation Document March 2000 THE NATURE CONSERVANCY and the SOUTHERN APPALACHIAN FOREST COALITION Southern Blue Ridge Ecoregional Conservation Plan Summary and Implementation Document Citation: The Nature Conservancy and Southern Appalachian Forest Coalition. 2000. Southern Blue Ridge Ecoregional Conservation Plan: Summary and Implementation Document. The Nature Conservancy: Durham, North Carolina. This document was produced in partnership by the following three conservation organizations: The Nature Conservancy is a nonprofit conservation organization with the mission to preserve plants, animals and natural communities that represent the diversity of life on Earth by protecting the lands and waters they need to survive. The Southern Appalachian Forest Coalition is a nonprofit organization that works to preserve, protect, and pass on the irreplaceable heritage of the region’s National Forests and mountain landscapes. The Association for Biodiversity Information is an organization dedicated to providing information for protecting the diversity of life on Earth. ABI is an independent nonprofit organization created in collaboration with the Network of Natural Heritage Programs and Conservation Data Centers and The Nature Conservancy, and is a leading source of reliable information on species and ecosystems for use in conservation and land use planning. Photocredits: Robert D. Sutter, The Nature Conservancy EXECUTIVE SUMMARY This first iteration of an ecoregional plan for the Southern Blue Ridge is a compendium of hypotheses on how to conserve species nearest extinction, rare and common natural communities and the rich and diverse biodiversity in the ecoregion. The plan identifies a portfolio of sites that is a vision for conservation action, enabling practitioners to set priorities among sites and develop site-specific and multi-site conservation strategies. -
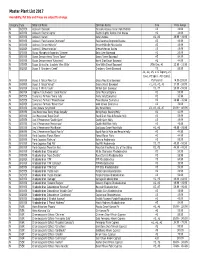
Master Plant List 2017.Xlsx
Master Plant List 2017 Availability, Pot Size and Prices are subject to change. Category Type Botanical Name Common Name Size Price Range N BREVER Azalea X 'Cascade' Cascade Azalea (Glenn Dale Hybrid) #3 49.99 N BREVER Azalea X 'Electric Lights' Electric Lights Double Pink Azalea #2 44.99 N BREVER Azalea X 'Karen' Karen Azalea #2, #3 39.99 - 49.99 N BREVER Azalea X 'Poukhanense Improved' Poukhanense Improved Azalea #3 49.99 N BREVER Azalea X 'Renee Michelle' Renee Michelle Pink Azalea #3 49.99 N BREVER Azalea X 'Stewartstonian' Stewartstonian Azalea #3 49.99 N BREVER Buxus Microphylla Japonica "Gregem' Baby Gem Boxwood #2 29.99 N BREVER Buxus Sempervirens 'Green Tower' Green Tower Boxwood #5 64.99 N BREVER Buxus Sempervirens 'Katerberg' North Star Dwarf Boxwood #2 44.99 N BREVER Buxus Sinica Var. Insularis 'Wee Willie' Wee Willie Dwarf Boxwood Little One, #1 13.99 - 21.99 N BREVER Buxus X 'Cranberry Creek' Cranberry Creek Boxwood #3 89.99 #1, #2, #5, #15 Topiary, #5 Cone, #5 Spiral, #10 Spiral, N BREVER Buxus X 'Green Mountain' Green Mountain Boxwood #5 Pyramid 14.99-299.99 N BREVER Buxus X 'Green Velvet' Green Velvet Boxwood #1, #2, #3, #5 17.99 - 59.99 N BREVER Buxus X 'Winter Gem' Winter Gem Boxwood #5, #7 59.99 - 99.99 N BREVER Daphne X Burkwoodii 'Carol Mackie' Carol Mackie Daphne #2 59.99 N BREVER Euonymus Fortunei 'Ivory Jade' Ivory Jade Euonymus #2 35.99 N BREVER Euonymus Fortunei 'Moonshadow' Moonshadow Euonymus #2 29.99 - 35.99 N BREVER Euonymus Fortunei 'Rosemrtwo' Gold Splash Euonymus #2 39.99 N BREVER Ilex Crenata 'Sky Pencil'