Amphibians and Reptiles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caudata: Hynobiidae): Heterochronies and Reductions
65 (1): 117 – 130 © Senckenberg Gesellschaft für Naturforschung, 2015. 4.5.2015 Development of the bony skeleton in the Taiwan salamander, Hynobius formosanus Maki, 1922 (Caudata: Hynobiidae): Heterochronies and reductions Anna B. Vassilieva 1 *, June-Shiang Lai 2, Shang-Fang Yang 2, Yu-Hao Chang 1 & Nikolay A. Poyarkov, Jr. 1 1 Department of Vertebrate Zoology, Biological Faculty, Lomonosov Moscow State University, Leninskiye Gory, GSP-1, Moscow 119991, Russia — 2 Department of Life Science, National Taiwan Normal University, 88, Sec. 4 Tingchou Rd., Taipei 11677, Taiwan, R.O.C. — *Cor- responding author; vassil.anna(at)gmail.com Accepted 19.ii.2015. Published online at www.senckenberg.de / vertebrate-zoology on 4.v.2015. Abstract The development of the bony skeleton in a partially embryonized lotic-breeding salamander Hynobius formosanus is studied using the ontogenetic series from late embryos to postmetamorphic juveniles and adult specimen. Early stages of skull development in this spe- cies are compared with the early cranial ontogeny in two non-embryonized lentic-breeding species H. lichenatus and H. nigrescens. The obtained results show that skeletal development distinguishes H. formosanus from other hynobiids by a set of important features: 1) the reduction of provisory ossifications (complete absence of palatine and reduced state of coronoid), 2) alteration of a typical sequence of ossification appearance, namely, the delayed formation of vomer and coronoid, and 3) the absence of a separate ossification center of a lacrimal and formation of a single prefrontolacrimal. These unique osteological characters in H. formosanus are admittedly connected with specific traits of its life history, including partial embryonization, endogenous feeding until the end of metamorphosis and relatively short larval period. -

BULLETIN Chicago Herpetological Society
BULLETIN of the Chicago Herpetological Society Volume 52, Number 5 May 2017 BULLETIN OF THE CHICAGO HERPETOLOGICAL SOCIETY Volume 52, Number 5 May 2017 A Herpetologist and a President: Raymond L. Ditmars and Theodore Roosevelt . Raymond J. Novotny 77 Notes on the Herpetofauna of Western Mexico 16: A New Food Item for the Striped Road Guarder, Conophis vittatus (W. C. H. Peters, 1860) . .Daniel Cruz-Sáenz, David Lazcano and Bryan Navarro-Velazquez 80 Some Unreported Trematodes from Wisconsin Leopard Frogs . Dreux J. Watermolen 85 What You Missed at the April Meeting . .John Archer 86 Gung-ho for GOMO . Roger A. Repp 89 Herpetology 2017......................................................... 93 Advertisements . 95 New CHS Members This Month . 95 Minutes of the April 14 Board Meeting . 96 Show Schedule.......................................................... 96 Cover: The end of a battle between two Sonoran Desert Tortoises (Gopherus morafkai). Photograph by Roger A. Repp, Pima County, Arizona --- where the turtles are strong! STAFF Membership in the CHS includes a subscription to the monthly Bulletin. Annual dues are: Individual Membership, $25.00; Family Editor: Michael A. Dloogatch --- [email protected] Membership, $28.00; Sustaining Membership, $50.00; Contributing Membership, $100.00; Institutional Membership, $38.00. Remittance must be made in U.S. funds. Subscribers 2017 CHS Board of Directors outside the U.S. must add $12.00 for postage. Send membership dues or address changes to: Chicago Herpetological Society, President: Rich Crowley Membership Secretary, 2430 N. Cannon Drive, Chicago, IL 60614. Vice-president: Jessica Wadleigh Treasurer: Andy Malawy Manuscripts published in the Bulletin of the Chicago Herpeto- Recording Secretary: Gail Oomens logical Society are not peer reviewed. -

Griffith Park Wildlife Management Plan
GRIFFITH PARK WILDLIFE MANAGEMENT PLAN (FINAL) January 22, 2009 Monterey ensatina Ensatina eschsholtzi, photographed in Brush Canyon in August 2008 (D.S. Cooper) Report submitted to the Los Angeles Department of Recreation and Parks by Cooper Ecological Monitoring, Inc. Contract No. 2930. Daniel S. Cooper and Paul Mathewson Cooper Ecological Monitoring, Inc. 5850 W. 3rd St., #167 Los Angeles, CA 90036 www.cooperecological.com Contact: [email protected] TABLE OF CONTENTS 1. ACKNOWLEDGEMENTS . 4 2. EXECUTIVE SUMMARY (incl. Best Management Practices) . 4 3. INTRODUCTION . 7 3.1. Justification for Plan . 7 3.2 Audience . 8 3.3 History . 9 3.4 Setting . 10 3.5 Wildlife Management Plan Goals and Guiding Principles . 11 4. HABITAT DESCRIPTIONS . 12 4.1 Terrestrial Habitats . 15 4.2 Aquatic Habitats . 22 4.3 Urban Interface Zone . 23 5. SPECIES INFORMATION . 24 5.1 Special-status species . 25 5.2 Stewardship species . 34 6. WILDLIFE MANAGEMENT GOALS . 41 6.1 Promote native wildlife populations and habitats . 41 6.1.1 Identify and defend native vegetation and biological "hotspots" . 41 6.1.2 Clarify location and usage of wildlife corridors . 42 6.1.3 Identify restoration priorities (incl. site descriptions) . 44 6.2 Facilitate the collection of wildlife distribution and ecological information . 51 6.3 Minimize human-wildlife conflict . 52 2 6.3.1 Strengthen law enforcement . 52 6.3.2 Consult with regulatory agencies . 52 6.3.3 Reduce "edge effects" . 54 6.3.4 Manage recreation to avoid conflicts with wildlife . 56 6.3.5 Reduce "passive wildlife feeding" . 58 6.3.6 Reduce use of rodenticides near wildland habitat . -
Resource Partitioning in Two Stream Salamanders, Dicamptodon Tenebrosus and Rhyacotriton Cascadae, from the Oregon Cascade Mountains
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/269579136 Resource Partitioning in Two Stream Salamanders, Dicamptodon tenebrosus and Rhyacotriton cascadae, from the Oregon Cascade Mountains Article in American Midland Naturalist · July 2014 DOI: 10.1674/0003-0031-172.1.191 CITATIONS READS 6 97 2 authors, including: R. Bruce Bury United States Geological Survey 123 PUBLICATIONS 2,940 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Herpetological Conservation and Biology View project Tailed frog phylogenetics View project All content following this page was uploaded by R. Bruce Bury on 09 February 2015. The user has requested enhancement of the downloaded file. Resource Partitioning in Two Stream Salamanders, Dicamptodon tenebrosus and Rhyacotriton cascadae, from the Oregon Cascade Mountains Author(s): Wynn W. CudmoreR. Bruce Bury Source: The American Midland Naturalist, 172(1):191-199. 2014. Published By: University of Notre Dame DOI: http://dx.doi.org/10.1674/0003-0031-172.1.191 URL: http://www.bioone.org/doi/full/10.1674/0003-0031-172.1.191 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. -

Snakes of Utah by Douglas C. Cox and Wilmer W. Tanner
Great Basin Naturalist Volume 56 Number 3 Article 16 7-26-1996 Snakes of Utah by Douglas C. Cox and Wilmer W. Tanner Andrew H. Barnum Dixie College, St. George, Utah Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Barnum, Andrew H. (1996) "Snakes of Utah by Douglas C. Cox and Wilmer W. Tanner," Great Basin Naturalist: Vol. 56 : No. 3 , Article 16. Available at: https://scholarsarchive.byu.edu/gbn/vol56/iss3/16 This Book Review is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Great Basin Naturalist 56(3), © 1996, pp. 283-285 BOOK REVIEW Snakes of Utah. Douglas C. Cox aud Wilmer would be more useful if a caption were shown W. Tanner; Mark Philbrick, photography. by the other photographs throughout the text, Monte L. Beau Life Science Museum, Brig e.g., the photo opposite page 1 aud those shown ham Young University, Provo, UT. 1996. on pages 3, 4, 5, 8. The herpetologist will $17.95 softcover. probably recognize these without caption, but, as stated, it's likely these specialists will not be Snakes of Utah, anticipated for some time, the primary users of the text. Identification of is finally available for distribution. This book snakes by these photographs may not be obvi let (92 total pages) includes all known species ous to most readers. -

Conservation Genetics of the Imperiled Striped Whipsnake in Washington, USA
Herpetological Conservation and Biology 15(3):597–610. Submitted: 9 March 2020; Accepted: 5 November 2020; Published: 16 December 2020. CONSERVATION GENETICS OF THE IMPERILED STRIPED WHIPSNAKE IN WASHINGTON, USA DAVID S. PILLIOD1,4, LISA A. HALLOCK2, MARK P. MILLER3, THOMAS D. MULLINS3, AND SUSAN M. HAIG3 1U.S. Geological Survey, Forest and Rangeland Ecosystem Science Center, 970 Lusk Street, Boise, Idaho 83706, USA 2Wildlife Program, Washington Department of Fish and Wildlife, 1111 Washington Street, Olympia, Washington 98504, USA 3U.S. Geological Survey, Forest and Rangeland Ecosystem Science Center, 3200 Southwest Jefferson Way, Corvallis, Oregon 97331, USA 4Corresponding author, email: [email protected] Abstract.—Conservation of wide-ranging species is aided by population genetic information that provides insights into adaptive potential, population size, interpopulation connectivity, and even extinction risk in portions of a species range. The Striped Whipsnake (Masticophis taeniatus) occurs across 11 western U.S. states and into Mexico but has experienced population declines in parts of its range, particularly in the state of Washington. We analyzed nuclear and mitochondrial DNA extracted from 192 shed skins, 63 muscle tissue samples, and one mouth swab to assess local genetic diversity and differentiation within and between the last known whipsnake populations in Washington. We then placed that information in a regional context to better understand levels of differentiation and diversity among whipsnake populations in the northwestern portion of the range of the species. Microsatellite data analyses indicated that there was comparable genetic diversity between the two extant Washington populations, but gene flow may be somewhat limited. We found moderate to high levels of genetic differentiation among states across all markers, including five microsatellites, two nuclear genes, and two mitochondrial genes. -

3.7 Biological Resources
3.7 BIOLOGICAL RESOURCES EXECUTIVE SUMMARY This section identifies major plant and animal resources within the City’s Planning Area and assesses the potential impacts of the proposed General Plan on biological resources with the understanding that certain resources, especially wildlife, are transitory and may potentially be present in a wide variety of areas regardless of previous records of observation. The City’s Planning Area consists of its incorporated boundaries and adopted Sphere of Influence (SOI). The County’s Planning Area consists of unincorporated land within the One Valley One Vision (OVOV) Planning Area boundaries that is located outside the City’s boundaries and the adopted SOI. The City and the County Planning Areas together comprise the OVOV Planning Area. A substantial portion of the area within the City has been developed. Species within the remaining natural areas are adapted to the Mediterranean climate of the region, in that they thrive in the cool, wet winters, and dry, hot summers typical of the area. Within the City boundaries, these areas include the Santa Clara River through the City; and portions of San Francisquito Canyon, Sand Canyon, Whitney Canyon, and Placerita Canyon. The major natural features of the City’s adopted SOI include the Liebre Mountains south of the National Forest boundary, including Cruzan Mesa and portions of Tick Canyon, Mint Canyon, Bouquet Canyon and San Francisquito Canyon; and the San Gabriel Mountains north of the National Forest boundary, including portions of Sand Canyon and -
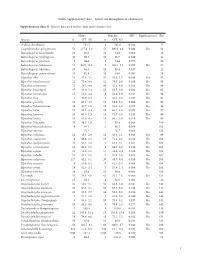
I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius -

Amphibian Assemblages in Zero-Order Basins in the Oregon Coast Range1
Color profile: Generic CMYK printer profile Composite Default screen 1452 Amphibian assemblages in zero-order basins in the Oregon Coast Range1 Chris D. Sheridan and Deanna H. Olson Abstract: Zero-order basins, extending from ridgelines to the initiation of first-order streams, were sampled in the Coast Range of Oregon to (i) characterize spatial distribution patterns of amphibian species and assemblages along lon- gitudinal and lateral gradients, and relative to three geomorphic surfaces (valleys, headmost areas, and slopes); and (ii) develop empirical species–habitat models. Unmanaged zero-order basins were hotspots for amphibian diversity, with significant differences across geomorphic gradients. Captures of riparian-associated amphibians were higher in valley areas, usually within2mofbasin center. Upland-associated amphibians were captured two times farther from basin centers than riparian-associated species, but highest densities occurred only 2–5 m from basin center. The most useful empirical models related captures of individual amphibian species to geomorphic, disturbance, moisture, and overstory variables. Ordination and indicator species analysis characterized geomorphic and other environmental gradients in am- phibian assemblages and suggested spatial compression of fluvial habitats and riparian-associated species in zero-order basins, in comparison with downstream areas. Our findings have implications for headwater areas managed to hedge risk to and uncertainty in amphibian persistence, namely in the delineation of zones with -

Ecol 483/583 – Herpetology Lab 2: Phylogeny Exercise, Amphibia Diversity 1: Urodela & Gymnophiona Spring 2010
Ecol 483/583 – Herpetology Lab 2: Phylogeny Exercise, Amphibia Diversity 1: Urodela & Gymnophiona Spring 2010 P.J. Bergmann & S. Foldi Lab objectives The objectives of today’s lab are to: 1. Continue to familiarize yourselves with local herps and their external anatomy. 2. Use this knowledge to collect morphological character data from specimens. 3. Reconstruct a phylogeny using your collected character data. 4. Be able to discuss phylogenetic concepts introduced in lecture in the context of the lab. 5. Familiarize yourselves with extant diversity of the Urodela and Gymnophiona. Collecting data from specimens and using it to reconstruct a phylogeny will reinforce your knowledge of local herps and accustom you to using the terminology from last week’s lab. During today’s lab you will also begin studying amphibian diversity. The lab will introduce the Urodela (salamanders) and Gymnophiona (caecilians). Tips for learning the material Take some time today to review some of the material that was covered last week. The same specimens are out to allow you to do this. Use the opportunity to quiz yourself or a partner by covering up species names and identifying specimens. Note which ones you find easy to ID and which ones give you trouble. Why do those give you troubles? Refine your criteria for differentiating them and take some more notes. However, do not spend too much time reviewing last week’s material – there are new things to cover as well. Today’s phylogeny exercise will help you to reinforce some of this material, at least for the lizards, which will be the focus of that exercise. -

Final Lower Rio Grande Valley and Santa Ana National Wildlife
Final Lower Rio Grande Valley and Santa Ana National Wildlife Refuges Comprehensive Conservation Plan September 1997 (Reprint March 1999) U.S. Fish and Wildlife Service U.S. Department of the Interior Cover Artwork by Brian Cobble Table of Contents VISION........................................................................................................................................... 5 Executive Summary................................................................................................................... 6 1.0 Introduction and Regional Setting................................................................................. 8 1.1 LRGV Challenges............................................................................................... 8 2.0 Planning Perspectives and Considerations................................................................ 9 2.1 National Wildlife Refuge System ................................................................... 9 2.2 The Service & Ecosystem Management ...................................................... 9 2.3 Refuge Complex and Management Districts........................................... 10 2.4 Laguna Atascosa NWR -- A Partner with LRGV NWR............................ 10 2.5 Planning Perspectives.................................................................................... 10 2.6 The Issues.......................................................................................................... 11 2.7 The Need for Action........................................................................................ -

Environmental Factors Potentially Affecting Eurycea Naufragia
Report to the Williamson County Conservation Foundation Review of Research Literature Related to the Biology, Evolution, and Conservation of Georgetown Salamander, Eurycea naufragia Benjamin A. Pierce and Ashley Wall Department of Biology Southwestern University Georgetown, Texas 78626 June 22, 2011 1 The Georgetown Salamander The Georgetown salamander, Eurycea naufragia, is a spring and cave-dwelling salamander restricted to the San Gabriel River drainage of Williamson County, Texas. The species is known from only 15 sites occurring along the major tributaries of the upper San Gabriel River (South, Middle, and North forks and Berry Creek; Figure 1). At some of these sites, salamanders have not been observed in recent years and access to all sites is not available. All but two of the known sites are on privately-owned land. The entire range of the species occurs within the immediate vicinity of Georgetown, Texas, an area that is undergoing rapid urbanization (Figure 2), and nearly all known populations are at risk from urban development. The Georgetown salamander has been included as a candidate for listing as an endangered species (US Fish and Wildlife Service, 2001) but is not currently protected by federal or state regulation. Candidate species are given a priority listing by the US Fish and Wildlife Service, which ranges from 1 to 12 and indicates the magnitude and immediacy of threats they face and their taxonomic uniqueness, with higher priority assigned to lower numbers. Largely because of the implementation of the Williamson County Regional Habitat Conservation Plan, the US Fish and Wildlife Service in 2008 reduced the listing priority number of the Georgetown salamander from 2 to 8 (US Fish and Wildlife Service, 2008).