Avian Influenza
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of the Birds of Christmas Island and Cocos (Keeling) Islands
Checklist of the Birds of Christmas Island and Cocos (Keeling) Islands Christmas Island Emerald Dove feathers R.E. Johnstone and J.C. Darnell Collections and Research, Western Australian Museum, Kew Street, Welshpool, WA 6106 †June 2021 ____________________________________ This checklist covers Christmas and Cocos (Keeling) Islands in the tropical eastern Indian Ocean and their surrounding seas. Christmas Island lies 290 km south of Java (Indonesia) and 1,400 km north-west of Western Australia. It is a small uplifted volcanic island (137 km² in area) and is administered by Western Australia as an External Territory of Australia. The Cocos (Keeling) Islands lie 970 km west of Christmas Island and 2,100 km north-west of Western Australia. They comprise two low-lying atolls, a large southern atoll (Cocos) consisting of about 26 islands around a horseshoe-shaped lagoon and a smaller North Keeling Island lying 25 km to the north. They cover a land area of about 14 km² and are also administered by Western Australia as an External Territory of Australia. The main aim of this work is to provide an up-to-date checklist of the birds of this region to include the large number of additional species that have been recorded on these islands since the publication of the Annotated Checklists of Christmas Island and Cocos (Keeling) Islands in 2004 (Johnstone and Darnell Appendix A and B in: Handbook of Western Australian Birds Volume II) and the more recent review of the birds of Christmas Island (James and McAllan 2014 Australian Field Ornithology Suppl. 31). Criterion for inclusion of a species or subspecies on the list is, in most cases, supported by tangible evidence i.e. -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

A 2010 Supplement to Ducks, Geese, and Swans of the World
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Ducks, Geese, and Swans of the World by Paul A. Johnsgard Papers in the Biological Sciences 2010 The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/biosciducksgeeseswans Part of the Ornithology Commons Johnsgard, Paul A., "The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World" (2010). Ducks, Geese, and Swans of the World by Paul A. Johnsgard. 20. https://digitalcommons.unl.edu/biosciducksgeeseswans/20 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Ducks, Geese, and Swans of the World by Paul A. Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. The World’s Waterfowl in the 21st Century: A 200 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard Pages xvii–xxiii: recent taxonomic changes, I have revised sev- Introduction to the Family Anatidae eral of the range maps to conform with more current information. For these updates I have Since the 978 publication of my Ducks, Geese relied largely on Kear (2005). and Swans of the World hundreds if not thou- Other important waterfowl books published sands of publications on the Anatidae have since 978 and covering the entire waterfowl appeared, making a comprehensive literature family include an identification guide to the supplement and text updating impossible. -

The Avifauna of Lambusango Forest Reserve, Buton Island, South-East Sulawesi, with Additional Sightings from Southern Buton
FORKTAIL 28 (2012): 107–112 The avifauna of Lambusango Forest Reserve, Buton Island, south-east Sulawesi, with additional sightings from southern Buton T. E. MARTIN, D. J. KELLY, N. T. KEOGH, D. HERIYADI, H. A. SINGER & G. A. BLACKBURN Lambusango Forest Reserve occupies a large area of south-central Buton, the largest attendant island of Sulawesi, Indonesia. Buton is located off Sulawesi’s south-eastern peninsula and remains poorly known ornithologically. Bird surveys were undertaken in the reserve over eight eight-week long research seasons between June and August in 1999, 2001–2003, 2005, and 2008–2010. Variable radius circular- plot point counts were the primary census method, conducted as part of a long-term biodiversity monitoring programme in the reserve, although data were also collected from 840 mist-netting hours and approximately 2,560 hours of observational data. In total, 79 species were detected in the reserve, including 37 regional endemics (46.8% of the total avifaunal community) and four species considered by the IUCN to be globally threatened or Near Threatened. Additionally, a further 60 species (including two more Near Threatened species) were recorded in various habitats around southern Buton that were not detected in Lambusango Reserve, giving a total of 139 species records for the island. We believe that 51 of these species represent previously unpublished records for Buton. We present here a full account of our records from Lambusango Reserve and southern Buton, with additional details provided for threatened and Near Threatened species and new records of endemics. INTRODUCTION Lambusango Forest Reserve (5°10’–5°24’S 122°43’–123°07’E) is a 65,000 ha expanse of uninhabited tropical monsoon forest, Buton (formerly referred to as Butung) is the largest of Sulawesi’s encompassing much of south-central Buton. -

1 Introduction 3 Micro-Organisms
I NTRODUCTION 1 The term Microbiology is coined with 3 Greek words LEARNING OBJECTIVES (Mikro – small, bios - life, logos science). In other After studying the chapter the words it is the study of organisms of microscopic size students familiarize themselves (micro-organisms) including their culture, economic with the following concepts: importance, pathogenecity, etc. This study is Introducing the subject concerned with their form, structure, reproduction, Microbiology and physiology, metabolism and classification. It includes significance of micro‐ the changes which micro-organisms bring about in organisms other organisms and in nonliving matter and their Different varieties of distribution in nature, their effects on human beings microscopes their invention and on other animals and plants, their abilities to make and constructions along physical and chemical changes in our environment and with simplified ray diagrams their reactions to physical and chemical agents. This study revealed the fact that there are many a great Contribution of scientists for the development of number of very tiny life forms all about us everywhere microbiology too small to be seen usually by the naked eye. These Introducing various organisms are usually invisible to naked eye because Branches of Microbiology they are in micron size. Our eye fails to perceive any object that has a diameter less than 0.1 mm, so it is necessary to use microscope to see these tiny forms of life. However, some micro-organisms, particularly some eukaryotic microbes, are visible without microscopes. For example, bread molds and filamentous algae are studied by microbiologists, yet are visible to the naked eye, as are the two bacteria Thiomargarita and Epulopiscioum. -

The Waterbirds and Coastal Seabirds of Timor-Leste: New Site Records Clarifying Residence Status, Distribution and Taxonomy
FORKTAIL 27 (2011): 63–72 The waterbirds and coastal seabirds of Timor-Leste: new site records clarifying residence status, distribution and taxonomy COLIN R. TRAINOR The status of waterbirds and coastal seabirds in Timor-Leste is refined based on surveys during 2005–2010. A total of 2,036 records of 82 waterbird and coastal seabirds were collected during 272 visits to 57 Timor-Leste sites, and in addition a small number of significant records from Indonesian West Timor, many by colleagues, are included. More than 200 new species by Timor-Leste site records were collected. Key results were the addition of three waterbirds to the Timor Island list (Red-legged Crake Rallina fasciata, vagrant Masked Lapwing Vanellus miles and recent colonist and Near Threatened Javan Plover Charadrius javanicus) and the first records in Timor-Leste for three irregular visitors: Australian White Ibis Threskiornis molucca, Ruff Philomachus pugnax and Near Threatened Eurasian Curlew Numenius arquata. Records of two subspecies of Gull-billed Tern Gelochelidon nilotica, including the first confirmed records outside Australia of G. n. macrotarsa, were also of note. INTRODUCTION number of field projects in Timor-Leste, including an Important Bird Areas programme and a doctoral study (Trainor et al. 2007a, Timor Island lies at the interface of continental South-East Asia and Trainor 2010). The residence status and nomenclature for some Australia and consequently its resident waterbird and coastal seabird species listed in a fieldguide (Trainor et al. 2007b) and recent review avifauna is biogeographically mixed. Some of the most notable (Trainor et al. 2008) are clarified. Three new island records are findings of a Timor-Leste field survey during 2002–2004 were the documented and substantial new ecological data on distribution discovery of resident breeding populations of the essentially Australian and habitat use are included. -

The Asian Waterbird Census 2008-2015: Results of Coordinated Counts in Asia and Australasia
The Asian Waterbird Census 2008-2015: Results of coordinated counts in Asia and Australasia Taej Mundkur, Tom Langendoen and Doug Watkins © Wetlands International 2017 Pages from this publication may be reproduced freely for educational, journalistic, and other non- commercial purposes. Prior permission must be given for all other forms of reproduction. Full credit must always be given to the copyright holder. Taej Mundkur1, Tom Langendoen2 and Doug Watkins3 1 International Waterbird Census Coordinator, Wetlands International 2 International Waterbird Census Data Manager, Wetlands International 3 Chair, EAAFP Monitoring Taskforce; Chair, Australasian Wader Studies Group (a special interest group of BirdLife Australia); Associate Expert, Wetlands International This publication should be cited as follows: Mundkur, T., Langendoen, T. and Watkins, D. (eds.) 2017. The Asian Waterbird Census 2008-2015 - results of coordinated counts in Asia and Australasia. Wetlands International, Ede. Cover photo: Black-tailed Godwit, © Sudheera Bandara The designations of geographical entities in this publication, and the presentation of material, do not imply the expression of any opinion whatsoever on the part of Wetlands International concerning the legal status of any country, territory, or area or its authorities, or concerning the delimitation of its frontiers or boundaries. The Asian Waterbird Census 2008-2015 - results of coordinated counts in Asia and Australasia The Asian Waterbird Census is supported by a network of volunteers and coordinated by the following organisations and agencies that work closely with Wetlands International. See www.wetlands.org/our-network/iwc-coordinators for full details Page 2 The Asian Waterbird Census 2008-2015 - results of coordinated counts in Asia and Australasia Contents Acknowledgements ................................................................................................................................ -
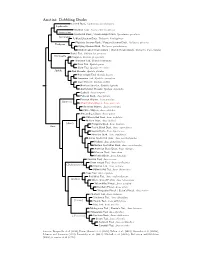
Anatini Tree
Anatini: Dabbling Ducks Crested Duck, Lophonetta specularioides Lophonetta Brazilian Teal, Amazonetta brasiliensis Amazonetta Spectacled Duck / Bronze-winged Duck, Speculanas specularis Speculanas Falkland Steamer-Duck, Tachyeres brachypterus Flightless Steamer-Duck / Fuegian Steamer-Duck, Tachyeres pteneres Tachyeres Flying Steamer-Duck, Tachyeres patachonicus White-headed Steamer-Duck / Chubut Steamer-Duck, Tachyeres leucocephalus Baikal Teal, Sibirionetta formosa Sibirionetta Garganey, Spatula querquedula Hottentot Teal, Spatula hottentota Puna Teal, Spatula puna Silver Teal, Spatula versicolor Spatula Red Shoveler, Spatula platalea Blue-winged Teal, Spatula discors Cinnamon Teal, Spatula cyanoptera Cape Shoveler, Spatula smithii Northern Shoveler, Spatula clypeata Australasian Shoveler, Spatula rhynchotis Gadwall, Anas strepera Falcated Duck, Anas falcata Eurasian Wigeon, Anas penelope (Mareca) ?Amsterdam Wigeon, Anas marecula American Wigeon, Anas americana Chiloe Wigeon, Anas sibilatrix African Black Duck, Anas sparsa Yellow-billed Duck, Anas undulata Meller’s Duck, Anas melleri (Anas) Philippine Duck, Anas luzonica Anas Pacific Black Duck, Anas superciliosa Laysan Duck, Anas laysanensis Hawaiian Duck, Anas wyvilliana Indian Spot-billed Duck, Anas poecilorhyncha Mallard, Anas platyrhynchos Eastern Spot-billed Duck, Anas zonorhyncha American Black Duck, Anas rubripes Mexican Duck, Anas diazi Mottled Duck, Anas fulvigula Eurasian Teal, Anas crecca Green-winged Teal, Anas carolinensis (Dafilonettion) ?Andean Teal, Anas andium Yellow-billed -

Reprinted from the Remedy: Robert Koch, Arthur Conan Doyle, and the Quest To
Reprinted from The Remedy: Robert Koch, Arthur Conan Doyle, and the Quest to Cure Tuberculosis by arrangement with Gotham Books, a member of Penguin Group (USA) LLC, A Penguin Random House Company. Copyright Thomas Goetz, 2014. On July 31, 1881, with the triumph of Pouilly‑le‑Fort spreading through Europe, Pasteur crossed the English Channel bound for London. His destination was St. James’s Palace and the Seventh International Medical Congress. Though there had been previous grand meetings on medicine, this seventh congress promised something of historical proportion. It seemed perfectly timed to capture a new passion for the potential of science. The British Medical Journal, its hometown pride evident, was effusive: It is always possible to exaggerate the greatness of events, as it is of monuments, to which we are in too close a proximity. .The mere fact of the meeting together in such unprecedented06 numbers of the leading powers engaged in the study and practice of medicine and the pursuit of collateral scientific work, has been a circumstance of which the influence in the future cannot but be long and deeply felt, and of which the present results are as interesting as they have been delightful. More than three thousand scientists attended from seventy countries— from “every land in which scientific medicine is practiced,” as The Lancet described it. The delegates were entertained as if they were dignitaries, feted by London’s most prominent citizens, including the lord mayor of London and the Baroness Burdett- Coutts. Pasteur was there, to discuss his spectacular work on the anthrax vaccine, as was Koch, who spoke about some remarkable laboratory techniques he’d developed at his new quarters in Berlin. -

Endemic Fauna of Andaman and Nicobar Islands Bay of Bengal
Endemic Fauna of Andaman and Nicobar Islands Bay of Bengal D.V. Rao, Kailash Chandra* and Kamla Devi** Freshwater Biology Regional Centre, Zoological Survey of India, Hyderabad-50004B 'Zoological Survey of India, M-Block, New Alipore, Kolkata 'Zoological Survey of India, Andaman and Nicobar Regional Centre, Port Blair Edited by the Director, Zoological Survey of India, Kolkata Zoological Survey of India Kolkata 1 Citation Rao, D.V., Kailash Chandra and Kamala Devi (2013). Endemic Animals of Andaman and Nicobar Islands, 182pp. E-Publication : September, 2013 ISBN: 978-81-8171-351-3 © Government of India, 2013 Published at the publication Division by the Director, Zoological Survey of India, M-Block, New Alipore, Kolkata - 700053 2 CONTENTS Page No. Introduction 4 Systematic list of Endemic fauna ...... 7 Mammals 24 Birds 28 Reptiles 46 Amphibia 51 Fishes 52 Molluscas 53 Earthworms 85 Crustaceans 86 Pycnogonida ........... 88 Insects 88 Arachnida 145 Chilopoda 146 Sponges 147 Soft & stinging corals 149 Spiny Crown Worms .......... .. 151 Protozoans 151 Meiofauna 152 Discussion 156 Summary 158 Acknowledgements 158 References 158 3 INTRODUCTION The Andaman and Nicobar Archipelago situated between 6°45' Nand 30°30' N lat. and 90°20' E and 93°56' E long. in the Bay of Bengal spread over a linear distance of over 550 km. comprises of over 350 islands, islets and rock outcrops including two out lying volcanic islands - Barren and Narcondam, are the summits of submarine mountain range that extends from the Eastern Himalaya along Arakan Voma of lower Myanmar in the north to Sumatra and lesser Sundas in the south. The total land area of the islands is about 8,293 sq km with a coastline of 1,962 km. -

Cavity-Nesting Ducks: Why Woodpeckers Matter Janet Kear
Cavity-nesting ducks: why woodpeckers matter Janet Kear Goosander Mergus merganser Rosemary Watts/Powell ABSTRACT This paper poses a number of related questions and suggests some answers.Why were there no resident tree-hole-nesting ducks in Britain until recently? Why is the Black Woodpecker Dryocopus martius not found in Britain? Could the supply of invertebrate food items, especially in winter, be responsible for the distribution of woodpeckers in western Europe? It is concluded that, in Europe, only the Black Woodpecker can construct holes large enough for ducks to nest in, and that this woodpecker does not occur in Britain & Ireland because of the absence of carpenter ants Campanotus. A plea is made for the global conservation of dead and dying timber, since it is vital for the biodiversity of plants, insects, woodpeckers and ducks. irdwatchers, at least in Britain, tend to surface-feeding ducks (Anatini), including think of ducks as nesting in the open and those which we used to call ‘perching ducks’, Bon the ground, but cavity-nesting is actu- and in the seaducks (Mergini). ally normal, if not obligatory, in almost one- Because ducks are incapable of making holes third of the 162 members of the wildfowl family for themselves, however, they must rely on (Anatidae). Geffen & Yom-Tov (2001) suggested natural agents for the construction of nest cavi- that hole-nesting has evolved independently at ties, and this dependence has profound implica- least three times within the group. I think that it tions for their lifestyles, breeding productivity is more likely to have arisen five or six times; and conservation. -

Naturally Hypernatural
ANTENNAE ISSUE 34 – WINTER 2015 ISSN 1756-9575 Naturally Hypernatural Suzanne Anker – Petri[e]’s Panopolis / Laura Ballantyne-Brodie – Earth System Ethics / Janet Gibbs – A Step on the Sun / Henry Sanchez – The English Kills Project / Steve Miller and Adam Stennett – Artist Survival Shack / Joe Mangrum – Sand Paintings / Tarah Rhoda and Nancy Chunn – Chicken Little and the Culture of Fear / Sarah E Durand – Newtown Creek ANTENNAE The Journal of Nature in Visual Culture Editor in Chief Giovanni Aloi – School of the Art Institute of Chicago, Sotheby’s Institute of Art London and New York, Tate Galleries Academic Board Advisory Board Steve Baker – University of Central Lancashire Rod Bennison Ron Broglio – Arizona State University Helen J. Bullard Matthew Brower – University of Toronto Claude d’Anthenaise Eric Brown – University of Maine at Farmington Lisa Brown Carol Gigliotti – Emily Carr University of Art and Design in Vancouver Chris Hunter Donna Haraway – University of California, Santa Cruz Karen Knorr Susan McHugh – University of New England Susan Nance Brett Mizelle – California State University Andrea Roe Claire Molloy – Edge Hill University David Rothenberg Cecilia Novero – University of Otago Angela Singer Jennifer Parker-Starbuck – Roehampton University Mark Wilson & Bryndís Snaebjornsdottir Annie Potts – University of Canterbury Ken Rinaldo – Ohio State University Nigel Rothfels – University of Wisconsin Jessica Ullrich – Friedrich-Alexander-Universität Erlangen-Nürnberg Andrew Yang – School of the Art Institute of Chicago Global Contributors Antennae (founded in 2006) is the international, peer reviewed, academic Sonja Britz journal on the subject of nature in contemporary art. Its format and Tim Chamberlain contents are inspired by the concepts of 'knowledge transfer' and 'widening Conception Cortes participation'.