CORPORATE PRESENTATION Q3 2020 Forward-Looking Statements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MECHANISMS in ENDOCRINOLOGY: Novel Genetic Causes of Short Stature
J M Wit and others Genetics of short stature 174:4 R145–R173 Review MECHANISMS IN ENDOCRINOLOGY Novel genetic causes of short stature 1 1 2 2 Jan M Wit , Wilma Oostdijk , Monique Losekoot , Hermine A van Duyvenvoorde , Correspondence Claudia A L Ruivenkamp2 and Sarina G Kant2 should be addressed to J M Wit Departments of 1Paediatrics and 2Clinical Genetics, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, Email The Netherlands [email protected] Abstract The fast technological development, particularly single nucleotide polymorphism array, array-comparative genomic hybridization, and whole exome sequencing, has led to the discovery of many novel genetic causes of growth failure. In this review we discuss a selection of these, according to a diagnostic classification centred on the epiphyseal growth plate. We successively discuss disorders in hormone signalling, paracrine factors, matrix molecules, intracellular pathways, and fundamental cellular processes, followed by chromosomal aberrations including copy number variants (CNVs) and imprinting disorders associated with short stature. Many novel causes of GH deficiency (GHD) as part of combined pituitary hormone deficiency have been uncovered. The most frequent genetic causes of isolated GHD are GH1 and GHRHR defects, but several novel causes have recently been found, such as GHSR, RNPC3, and IFT172 mutations. Besides well-defined causes of GH insensitivity (GHR, STAT5B, IGFALS, IGF1 defects), disorders of NFkB signalling, STAT3 and IGF2 have recently been discovered. Heterozygous IGF1R defects are a relatively frequent cause of prenatal and postnatal growth retardation. TRHA mutations cause a syndromic form of short stature with elevated T3/T4 ratio. Disorders of signalling of various paracrine factors (FGFs, BMPs, WNTs, PTHrP/IHH, and CNP/NPR2) or genetic defects affecting cartilage extracellular matrix usually cause disproportionate short stature. -

Significant Absorption of Topical Tacrolimus in 3 Patients with Netherton Syndrome
OBSERVATION Significant Absorption of Topical Tacrolimus in 3 Patients With Netherton Syndrome Angel Allen, MD; Elaine Siegfried, MD; Robert Silverman, MD; Mary L. Williams, MD; Peter M. Elias, MD; Sarolta K. Szabo, MD; Neil J. Korman, MD, PhD Background: Tacrolimus is a macrolide immunosup- limus in organ transplant recipients. None of these pressant approved in oral and intravenous formulations patients developed signs or symptoms of toxic effects of for primary immunosuppression in liver and kidney trans- tacrolimus. plantation. Topical 0.1% tacrolimus ointment has re- cently been shown to be effective in atopic dermatitis for Conclusions: Patients with Netherton syndrome have children as young as 2 years of age, with minimal sys- a skin barrier dysfunction that puts them at risk for in- temic absorption. We describe 3 patients treated with topi- creased percutaneous absorption. The Food and Drug Ad- cal 0.1% tacrolimus who developed significant systemic ministration recently approved 0.1% tacrolimus oint- absorption. ment for the treatment of atopic dermatitis. Children with Netherton syndrome may be misdiagnosed as having Observation: Three patients previously diagnosed as atopic dermatitis. These children are at risk for marked having Netherton syndrome were treated at different cen- systemic absorption and associated toxic effects. If topi- ters with 0.1% tacrolimus ointment twice daily. Two pa- cal tacrolimus is used in this setting, monitoring of se- tients showed dramatic improvement. All patients were rum tacrolimus levels is essential. found to have tacrolimus blood levels within or above the established therapeutic trough range for oral tacro- Arch Dermatol. 2001;137:747-750 ETHERTON syndrome is taneous absorption of the drug, with serum an autosomal recessive levels well above the therapeutic range. -

Chronic Diarrhea in an Adolescent Girl with a Genetic Skin Condition
PHOTO CHALLENGE Chronic Diarrhea in an Adolescent Girl With a Genetic Skin Condition Lucia Liao, BS; Andrea Zaenglein, MD; Galen T. Foulke, MD A 17-year-old adolescent girl visited our clinic to establish care for her genetic skin condition. She exhibited red scaly plaques and patches over much of the body surface area consistent with atopic dermatitis but also had areas on the trunk with serpiginous red plaques with scale on the leading and trailingcopy edges. She also noted fragile hair with sparse eyebrows. The patient reported that she had experienced chronic diarrhea and abdominal pain since childhood. She asked if it couldnot be related to her genetic condition. WHAT’S THE DIAGNOSIS? a. dyskeratosis follicularis (Darier disease) b. elastosis perforans serpiginosa Doc. erythema marginatum d. Netherton syndrome e. subacute cutaneous lupus erythematosus PLEASE TURN TO PAGE E19 FOR THE DIAGNOSIS CUTIS Ms. Liao is from Pennsylvania State University College of Medicine, Hershey. Drs. Zaenglein and Foulke are from the Department of Dermatology, Pennsylvania State Medical Center, Hershey. Dr. Zaengelin also is from the Department of Pediatrics. The authors report no conflict of interest. Correspondence: Galen T. Foulke, MD, 500 University Dr HU14, Hershey, PA 17033 ([email protected]). E18 I CUTIS® WWW.MDEDGE.COM/DERMATOLOGY Copyright Cutis 2020. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. PHOTO CHALLENGE DISCUSSION THE DIAGNOSIS: Netherton Syndrome -

ESID Registry – Working Definitions for Clinical Diagnosis of PID
ESID Registry – Working Definitions for Clinical Diagnosis of PID These criteria are only for patients with no genetic diagnosis*. *Exceptions: Atypical SCID, DiGeorge syndrome – a known genetic defect and confirmation of criteria is mandatory Available entries (Please click on an entry to see the criteria.) Page Acquired angioedema .................................................................................................................................................................. 4 Agammaglobulinaemia ................................................................................................................................................................ 4 Asplenia syndrome (Ivemark syndrome) ................................................................................................................................... 4 Ataxia telangiectasia (ATM) ......................................................................................................................................................... 4 Atypical Severe Combined Immunodeficiency (Atypical SCID) ............................................................................................... 5 Autoimmune lymphoproliferative syndrome (ALPS) ................................................................................................................ 5 APECED / APS1 with CMC - Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) .................. 5 Barth syndrome ........................................................................................................................................................................... -

Hereditary Palmoplantar Keratoderma "Clinical and Genetic Differential Diagnosis"
doi: 10.1111/1346-8138.13219 Journal of Dermatology 2016; 43: 264–274 REVIEW ARTICLE Hereditary palmoplantar keratoderma “clinical and genetic differential diagnosis” Tomo SAKIYAMA, Akiharu KUBO Department of Dermatology, Keio University School of Medicine, Tokyo, Japan ABSTRACT Hereditary palmoplantar keratoderma (PPK) is a heterogeneous group of disorders characterized by hyperkerato- sis of the palm and the sole skin. Hereditary PPK are divided into four groups – diffuse, focal, striate and punctate PPK – according to the clinical patterns of the hyperkeratotic lesions. Each group includes simple PPK, without associated features, and PPK with associated features, such as involvement of nails, teeth and other organs. PPK have been classified by a clinically based descriptive system. In recent years, many causative genes of PPK have been identified, which has confirmed and/or rearranged the traditional classifications. It is now important to diag- nose PPK by a combination of the traditional morphological classification and genetic testing. In this review, we focus on PPK without associated features and introduce their morphological features, genetic backgrounds and new findings from the last decade. Key words: diffuse, focal, punctate, striate, transgrediens. INTRODUCTION psoriasis vulgaris confined to the palmoplantar area (Fig. 1b) are comparatively common and are sometimes difficult to Palmoplantar keratoderma (PPK) is a heritable or acquired dis- distinguish from hereditary PPK. A skin biopsy is essential in order characterized by abnormal hyperkeratotic thickening of diagnosing these cases. Lack of a family history is not neces- the palm and sole skin. In a narrow sense, PPK implies heredi- sarily evidence of an acquired PPK, because autosomal reces- tary PPK, the phenotype of which usually appears at an early sive PPK can appear sporadically from parent carriers and age. -

Anhidrotic Ectodermal Dysplasia and Immunodeficiency: the Role of NEMO E D Carrol, a R Gennery, T J Flood, G P Spickett, M Abinun
340 CASE REPORT Arch Dis Child: first published as 10.1136/adc.88.4.340 on 1 April 2003. Downloaded from Anhidrotic ectodermal dysplasia and immunodeficiency: the role of NEMO E D Carrol, A R Gennery, T J Flood, G P Spickett, M Abinun ............................................................................................................................. Arch Dis Child 2003;88:340–341 Streptococcus pneumoniae. We found that he had associated spe- Anhidrotic (hypohidrotic) ectodermal dysplasia associated cific antibody deficiency (SPAD), in particular antipolysaccha- with immunodeficiency (EDA-ID; OMIM 300291) is a ride antibody deficiency.1 He initially responded well to intra- newly recognised primary immunodeficiency caused by venous immunoglobulin (IVIg) replacement, but as one of the κ mutations in NEMO, the gene encoding nuclear factor B possible explanations for his SPAD was a maturational delay of κ κ (NF- B) essential modulator, NEMO, or inhibitor of B the immune system, this was stopped after two years and his γ kinase (IKK- ). This protein is essential for activation of the specific antibody production was reassessed. The original κ transcription factor NF- B, which plays an important role diagnosis was confirmed, as well as low IgG2 subclass level in human development, skin homoeostasis, and immunity. and very low specific antibody response to tetanus toxoid. He was recommenced on IVIg replacement, and at follow up at age 11 years he has remained free of major infections with no evidence of bronchiectasis on high resolution chest computer- e present an update on the first reported patient ised tomography (CT) scanning. However, his serum IgA 1 with EDA-ID syndrome subsequently shown to be remains very high and that of IgM is declining, suggestive of 2 Wcaused by NEMO mutation, and our current under- ongoing immune dysregulation (table 1). -
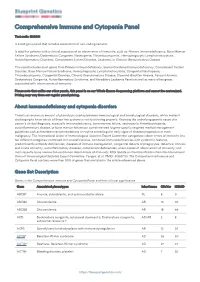
Blueprint Genetics Comprehensive Immune and Cytopenia Panel
Comprehensive Immune and Cytopenia Panel Test code: IM0901 Is a 642 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of an inborn error of immunity, such as, Primary Immunodeficiency, Bone Marrow Failure Syndrome, Dyskeratosis Congenita, Neutropenia, Thrombocytopenia, Hemophagocytic Lymphohistiocytosis, Autoinflammatory Disorders, Complement System Disorder, Leukemia, or Chronic Granulomatous Disease. This panel includes most genes from Primary Immunodeficiency, Severe Combined Immunodeficiency, Complement System Disorder, Bone Marrow Failure Syndrome, Hemophagocytic Lymphohistiocytosis, Congenital Neutropenia, Thrombocytopenia, Congenital Diarrhea, Chronic Granulomatous Disease, Diamond-Blackfan Anemia, Fanconi Anemia, Dyskeratosis Congenita, Autoinflammatory Syndrome, and Hereditary Leukemia Panels as well as many other genes associated with inborn errors of immunity. Please note that unlike our other panels, this panel is on our Whole Exome Sequencing platform and cannot be customized. Pricing may vary from our regular panel pricing. About immunodeficiency and cytopenia disorders There is an enormous amount of phenotypic overlap between immunological and hematological disorders, which makes it challenging to know which of these two systems is not functioning properly. Knowing the underlying genetic cause of a person’s clinical diagnosis, especially immunodeficiency, bone marrow failure, neutropenia, thrombocytopenia, autoinflammatory disease, or bone marrow failure can sometime -

ICHTHYOSIS FOCUS Vol
ICHTHYOSIS FOCUS Vol. 20, No. 1 A Quarterly Journal for Friends of F.I.R.S.T. Spring 2001 An Ichthyosis Update – Part II This is adapted from a talk given by Dr. Mary L. Williams at the Academy of Dermatology Annual Meeting in San Francisco in March, 2000 and edited by Rita Tanis. Genetic Heterogeneity Confusing enough? I find refuge in the nized. At least 3 genetic loci have been limited personal experience, and corrobo- “Rule of Heterogeneity”: When in Las implicated in causing these disorders, rated by others1, the classic lamellar Vegas, put your money on heterogeneity of using genetic linkage analysis, although ichthyosis (LI) phenotype with large genotypes (specific gene mutations) and only one gene, encoding the enzyme, ker- plate-like scales is typically associated phenotypes (how the gene is expressed; its atinocyte transglutaminase 1, has been with transglutaminase mutations, while clinical features). Corrollary 1 to the rule identified to date. This enzyme is unique to the nonbullous congenital ichthyosiform is: multiple genes may be found to produce epidermis, hence the skin is only abnormal erythroderma (CIE) phenotype with a a common phenotype. Corollary 2 is: mul- in these patients. The enzyme is involved finer, lighter scale pattern is not. tiple phenotypes will be associated with in crosslinking proteins, such as loricrin, to However, others have reported no correla- mutations affecting a single gene. form the cornified envelope. The cornified tion between TG1 mutations and CIE or The lamellar ichthyosis group of auto- envelope forms a shell that surrounds the LI phenotypes2, and the final word is not somal recessive, primary ichthyosis is an cells of the outer skin layers. -

Differential Diagnosis of Neonatal and Infantile Erythroderma
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Acta Dermatovenerol Croat 2007;15(3):178-190 REVIEW Differential Diagnosis of Neonatal and Infantile Erythroderma Lena Kotrulja1, Slobodna Murat-Sušić2, Karmela Husar2 1University Department of Dermatology and Venereology, Sestre milosrdnice University Hospital; 2University Department of Dermatology and Venereology, Zagreb University Hospital Center and School of Medicine, Zagreb, Croatia Corresponding author: SUMMARY Neonatal and infantile erythroderma is a diagnostic and Lena Kotrulja, MD, MS therapeutic challenge. Numerous underlying causes have been reported. Etiologic diagnosis of erythroderma is frequently difficult to University Department of Dermatology establish, and is usually delayed, due to the poor specificity of clinical and Venereology and histopathologic signs. Differential diagnosis of erythroderma is Sestre milosrdnice University Hospital a multi-step procedure that involves clinical assessment, knowledge of any relevant family history and certain laboratory investigations. Vinogradska 29 Immunodeficiency must be inspected in cases of severe erythroderma HR-10000 Zagreb with alopecia, failure to thrive, infectious complications, or evocative Croatia histologic findings. The prognosis is poor with a high mortality rate [email protected] in immunodeficiency disorders and severe chronic diseases such as Netherton’s syndrome. Received: June 14, 2007 KEY WORDS: erythroderma, neonatal, infantile, generalized Accepted: July 11, 2007 exfoliative dermatitis INTRODUCTION Erythroderma is defined as an inflammatory Neonatal and infantile erythroderma is a diag- skin disorder affecting total or near total body sur- nostic and therapeutic challenge. Erythrodermic face with erythema and/or moderate to extensive neonates and infants are frequently misdiagnosed scaling (1). It is a reaction pattern of the skin that with eczema and inappropriate topical steroid can complicate many underlying skin conditions at treatment can lead to Cushing syndrome. -

Two Siblings Affected by Netherton/Comèl Syndrome
Volume 25 Number 7| July 2019| Dermatology Online Journal || Case Presentation 25(7):8 Two siblings affected by Netherton/Comèl syndrome. Diagnostic pathology and description of a new SPINK5 variant C Schepis1, M Siragusa1, A Centofanti2, M Vinci3, F Calì3 Affiliations: 1Unit of Dermatology, Oasi Research Institute - IRCCS, Troina, Italy, 2Department of Biomedical and Dental Sciences and Morphofunctional Imaging, University of Messina, Messina, Italy, 3Laboratory of Molecular Genetics, Oasi Research Institute - IRCCS, Troina, Italy Corresponding Author: Carmelo Schepis MD, Oasi Research Institute - IRCCS, Troina, Italy, Via Conte Ruggero, 73, 94018 Troina (EN), Email: [email protected] particular sign on microscopic observation. Abstract Trichoscopic examinations, indeed, shows a swelling Netherton syndrome is a severe, autosomal recessive along the hair shaft, which looks like a fishing pole or form of ichthyosis associated with mutations in the a bamboo cane. A further optical microscopic SPINK5 gene encompassing three main clinical analysis shows trichorrhexis invaginata, the findings: 1) ichthyosiform dermatitis and/or pathognomonic sign of Netherton syndrome. ichthyosis linearis circumflexa, 2) hair shaft defects with peculiar “trichorrhexis invaginata” (bamboo pole hair) findings, 3) atopic dermatitis. We describe Case Synopsis two siblings affected by Netherton/Comèl syndrome We present two siblings affected by who were referred to our Center for Netherton/Comèl syndrome. The two were born to Genodermatosis. A diagnostic pathway and the unrelated parents and two elder sisters were not description of a new SPINK5 variant has been affected. determined for these two patients. A novel genetic A 13-year-old boy was referred to our center for a mutation has been found. -

The Genetics of Hair Shaft Disorders
CONTINUING MEDICAL EDUCATION The genetics of hair shaft disorders AmyS.Cheng,MD,a and Susan J. Bayliss, MDb,c Saint Louis, Missouri Many of the genes causing hair shaft defects have recently been elucidated. This continuing medical education article discusses the major types of hair shaft defects and associated syndromes and includes a review of histologic features, diagnostic modalities, and findings in the field of genetics, biochemistry, and molecular biology. Although genetic hair shaft abnormalities are uncommon in general dermatology practice, new information about genetic causes has allowed for a better understanding of the underlying pathophysiologies. ( J Am Acad Dermatol 2008;59:1-22.) Learning objective: At the conclusion of this article, the reader should be familiar with the clinical presentation and histologic characteristics of hair shaft defects and associated genetic diseases. The reader should be able to recognize disorders with hair shaft abnormalities, conduct appropriate referrals and order appropriate tests in disease evaluation, and select the best treatment or supportive care for patients with hair shaft defects. EVALUATION OF THE HAIR progresses via interactions with the mesenchymal For the student of hair abnormalities, a full review dermal papillae, leading to the formation of anagen of microscopic findings and basic anatomy can be hairs with complete follicular components, including found in the textbook Disorders of Hair Growth by sebaceous and apocrine glands.3 Elise Olsen,1 especially the chapter on ‘‘Hair Shaft Anagen hair. The hair shaft is composed of three Disorders’’ by David Whiting, which offers a thor- layers, called the medulla, cortex, and cuticle (Fig 1). ough review of the subject.1 The recognition of the The medulla lies in the center of the shaft and anatomic characteristics of normal hair and the effects contains granules with citrulline, an amino acid, of environmental factors are important when evalu- which is unique to the medulla and internal root ating a patient for hair abnormalities. -

Hair Loss in Children
702 ArchivesofDiseasein Childhood 1993; 68: 702-706 PERSONAL PRACTICE Hair loss in children Julian Verbov There are many causes of hair loss in children Causes ofhair loss in children and I shall mention some of these (table), but I Aplasia cutis/other scarring alopecias will deal in more detail with alopecia areata and Sebaceous naevus with children who pull their hair. Hereditary Telogen effluvium Normal hair growth in children has been Chemical reviewed recently.' Endocrine Nutritional Ringworm Alopecia areata Trauma: Aplasia cutis and other scarring alopecias Traction Congenital absence of skin (aplasia cutis) Loose anagen Shaft defects presents on the scalp as one or more non- Pulled hair inflammatory well defined oval or circular ulcers, crusted areas (fig 1) or as scars. Lesions usually occur over the vertex in or adjacent to the midline and may involve skin only or occasion- surgical operation, severe bacterial, viral, or ally may extend deeply to bone and dura. fungal infections and uncommon conditions such Complications include secondary infection, as chronic folliculitis, sarcoidosis, or fronto- bleeding, and meningitis with deeper lesions. parietal morphoea. Occasionally other developmental defects are present and there may be a family history of aplasia cutis.2 Sebaceous naevus This is an uncommon, usually small, congenital lesion containing both epidermal and dermal PROGNOSIS AND MANAGEMENT elements. Most common over the scalp and Most lesions are superficial and heal over a usually single, it appears as a smooth slightly period ofmany weeks, leaving an area ofscarring raised hairless waxy plaque, yellow orange in alopecia and this is how they often present. colour and linear or slightly oval in shape.