Download Article (Pdf)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
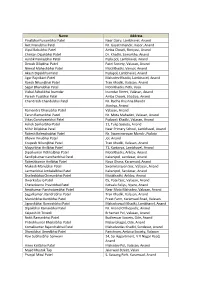
Members – List.Pdf
Name Address Pinalbhai Punambhai Patel Near Dairy, Lambhavel, Anand Axit Manubhai Patel Nr. Gayatrimandir, Kasor, Anand Vipul Babubhai Patel Amba Chowk, Boriyavi, Anand Chintan Dipakbhai Patel Dr. Khadki, Samarkha, Anand Hardik Pankajbhai Patel Pipla pol, Lambhavel, Anand Denish Dilipbhai Patel Patel Society, Valasan, Anand Nirmal Maheshbhai Patel Moti Khadki, Vansol, Anand Akash Dipakbhai Patel Piplapol, Lambhavel, Anand Jigar Rajnikant Patel Mahadev Khadki, Lambhavel, Anand Ronak Nikunjbhai Patel Tran Khadki, Valasan, Anand Sagar Bhanubhai Patel Moti Khadki, Petli, Vaso Vishal Ashokbhai Inamdar Inamdar Street, Valasan, Anand Paresh Pujabhai Patel Amba Chowk, Jitodiya, Anand Chandresh Chandubhai Patel Nr. Radha Krushna Mandir Jitodiya, Anand Ramendra Dhanjibhai Patel Valasan, Anand Tarun Ramanbhai Patel Nr. Mota Mahadev, Valasan, Anand Vikas Ganshyambhai Patel Piplavali Khadki, Valasan, Anand Ashok Sankarbhai Patel 11, Tulip Society, Anand Mihir Dilipbhai Patel Near Primary School, Lambhavel, Anand Rakesh Balendrabhai Patel Nr. Swaminarayan Mandir, Piplata Bhavin Vinubhai Patel Jol, Anand Krupesh Nikunjbhai Patel Tran Khadki, Valasan, Anand Mayurbhai Anilbhai Patel 71, Kartavya, Lambhavel, Anand Dipalkumar Vithhalbhai Patel Moti Khadki, Anklav, Anand. Sandipkumar Kanchanbhai Patel Kakanipol, sandesar, Anand Rakeshkumar Anilbhai Patel Nava Ghara, Karamsad, Anand Mukesh Manubhai Patel Swaminarayan Soc, Valasan, Anand Laxmanbhai Ambalalbhai Patel Kakanipol, Sandesar, Anand Shaileshbhai Chimanbhai Patel Motikhadki, Anklav, Anand Dwarkadas -

Kanu Patel (Kanaiyalal Fakirchand Patel) Th Born : 30 November, 1966
Kanu Patel (Kanaiyalal Fakirchand Patel) th Born : 30 November, 1966. Visnagar (Gujarat) Qualification : Art Teachers Diploma - 1984 (Centre First) Diploma in Painting - 1988 (Board First) Awards: • ‘Gaurav Purskar’ (Year 2010-11) Specially honoured by the Gujarat Lalit Kala Academy for the field of Painting. • Specially honoured by the Chief Minister of Gujarat for my services in the progress of the Nation in the field of Painting and Drama for year 2004. • Specially honoured by Visnagar Jaycees for my services in the progress of Nation in the field of Painting and Drama for year 2004. Best Painting : 78th Annual Exhibition , Indian Academy of fine Arts, Amritsar 2012 Graphic : 10th All India Art Contest, Nagpur 1996 Graphic : Gujarat State Lalit Kala Academy 1995 Graphic : 8th All India Art Contest, Nagpur 1994 Best Painting : Maha Koshal Kala Parishad, Raipur, M.P. 1993 Best Actor : “Khayal Bharmali” by Nica, Baroda 1993 Best Actor : “Ek Tha Gadha” by Nica and Hum 1991-93 Best Actor : “Khayal Bharmali” by Hum, Baroda 1992 Best Play : “Julus” by Nica, Baroda 1990 Best Actor : “Parmatmaka Kutta” by Nica, Baroda 1989 Best Painting : Annual Art Exhibition , Fine Arts College 1988 Best Painting : 16th Gujarat State Yuvak Mahotsav 1984 Exhibitions: One Man Show: 1991 N.D.D.B. (Boho Club), Anand 1993 Sursagar (Leicester) (UK) 1994 Ravishankar Raval Kala Bhavan, Ahmedabad 1997 Ravishankar Raval Kala Bhavan, Ahmedabad 1999 Elecon–CVM Public Garden, Vallabh Vidyanagar 2001 Ipcowala Santram College of Fine Arts, Vallabh Vidyanagar 2001 Welcome -

District Census Handbook, Kheda, Part XIII-A & B, Series-5
PARTS XIII-A & B ~:~ENS lJ S 1981 TOWN & VILLAGE DIRECTORY VILLAGE & TOWNWISE PRIMARY CENSUS SERIES-5 ABSTRACT GUJARAT DISTRICT CENSUS KHEDA HANDBOOK DISTRICT R. S. CHHAYA of the Indian Administrative Selvice Dir,cIOl' 0/ Census Op6Tlllions tJujetrat GUJARAT DISTRICT KHEDA 4 0 8 12 16 20 MILES I I I I S=t I I I I I 4 0 12 16 20 24 28 Kms ~ [l!!J VQUtlbh VldyonQgor PTO. e~~~n. CIJ~ • RH DB VithClI Ucfyognagor IN.A. A:ANAND > DISTRICT HEADQUARTERS. TALVKA HEADQUARTERS. DISTRICT BOUNDARY. TALUKA eOUNDARY NATIONAL HIGHWAY. NH. STATE HIGHWAY. SH 16 OTHER IMPORTANT ROAD .. o "S RAILWAY LINE WITH STATION, BROAD GAUGE.. [iiJ RAILWAY LINE WITH STATION,NARROW GAUGE. "II'~ II II RIVER AND STREAM .a ~ VILLAGE HAVING 5000 AND ABOVE Chunel \ S POPULATION WITH NAME. • URBAN AREA WITH POPULATION SIZE: o • ••••••• CLASS 1,II,llI,TV J V & VI. ••• POSOT AND TELEGRAPH OFFICE .. PTO DEGREE COLLEGE AND TECHNICAL tNSTITUT10N OAK BUNGALOW, REST HOUSE,TRAVELlERS DB. RH. TB, BUNGALOW, FOREST BUNGALOW AND F8, CANAL BUNGALOW .. .... C8 tN.A: INOUSTRIAL NOTIFIED AREA Kheda district's Co-operative Milk Producers Union Limited. Anand (po pularly known as Amul Dairy. Anand) not only gain much importance in the dairing development in our country but is well known to the rest of the world for milk as well as milk products. Thus, Kheda district is appearing on the map as leading dairy development Unit. It is located at Mogar. 7.5 kms. south of Anand on National Highway No.8. The divercification project plant (shown in the picture) is a high protein ready to eat Food Manufacturing Uhit dedicated to the cause of Infant Nutrition and distributing nutritious food everyday to about 3,70,000 children located in urban slums and rural) areas. -
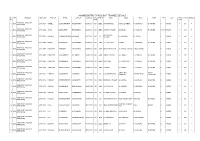
ANAND DISTRICT PASS out TRAINEE DETAILS Sr
ANAND DISTRICT PASS OUT TRAINEE DETAILS Sr. YRC TRADE_N SEAT_NO FIRST_N NAME LAST_N B_DATE PHY_ CASTE ADD1 ADD2 ADD3 ADD4 PIN ITI_N TRIA GTOTAL RESULT No. HAND L_NO ARMATURE & MOTOR 1 2010 216811001 GOHEL JAGDISHKUMAR MADHAVBHAI 01/06/1987 NO SEBC SUTHAR FALIU AT&PO:-DAHEMI TA:-BORSAD DI:-ANAND 0 VASAD 1 533 P REWINDING ARMATURE & MOTOR 2 2010 216811002 JADAV DILIPKUMAR BHANUBHAI 04/09/1988 NO SEBC GANPATI CHOWK AT:-ZILOD TA:-ANKLAV DI:-ANAND 388510 VASAD 1 514 P REWINDING ARMATURE & MOTOR OPP-BHARAT 3 2010 216811003 KALASVA RAVINDRAKUMAR KALUBHAI 07/11/1993 NO ST AT:-BORSAD DI:-ANAND 0 VASAD 1 543 P REWINDING SAWMILL ARMATURE & MOTOR 4 2010 216811004 MALEK IMRANKHAN ANAVARBHAI 07/06/1991 NO GEN AT:-VADI FALIA PAMOL TA:-BORSAD DI:-ANAND 0 VASAD 1 593 P REWINDING ARMATURE & MOTOR 5 2010 216811005 PADHIYAR AMBUBHAI CHIMANBHAI 01/06/1991 NO GEN BHAGVAN NAGAR AT:-NAPAD TALPAD TA&DI:-ANAND 0 VASAD 1 564 P REWINDING ARMATURE & MOTOR 6 2010 216811007 PADHIYAR JAGDISHBHAI NATUBHAI 23/05/1992 NO GEN AMBAV PARAMA AT:-AMBAV TA:-ANKLAV DI:-ANAND 0 VASAD 1 568 P REWINDING ARMATURE & MOTOR 7 2010 216811008 PADHIYAR MAHESHBHAI GOVINDBHAI 22/12/1990 NO SEBC MOTIPURA AT:-JOSHIKUVA TA:-ANKLAV DI:-ANAND 0 VASAD 1 549 P REWINDING ARMATURE & MOTOR 8 2010 216811010 PADHIYAR SANJAYKUMAR CHANDUBHAI 13/08/1993 NO GEN PARAMA AT:-AMBAV TA:-ANKLAV DI:-ANAND 0 VASAD 1 619 P REWINDING ARMATURE & MOTOR OPP-ALMAC TA&DI:- 9 2010 216811011 PARMAR ALPESHBHAI KANUBHAI 03/11/1990 NO SC A.23,GANHNAGAR CHHANI ROAD 0 VASAD 1 545 P REWINDING COMPANY VADODARA ARMATURE & MOTOR -

BHARAT SANCHAR NIGAM LIMITED (A Govt
1 BHARAT SANCHAR NIGAM LIMITED (A Govt. of India Enterprise) O/o GENERAL MANAGER TELECOM DISTRICT 2nd Floor, Door Sanchar Bhavan, Pij Road, NADIAD-387002 e-Tender No. KTD/MM/T 04/House Keeping Work/2015-‘16 TENDER DOCUMENT PART – A TECHNICAL BID HOUSE KEEPING WORK (Sweeping & Cleaning) FOR OFFICE BUILDINGS & EXCHANGE BUILDINGS FOR BORSAD, ANKLAV, PETLAD, SOJITRA, CAMBAY, TARAPUR, NADIAD, MAHUDHA, KHEDA, MEHMDABAD, BALASINOR, VIRPUR, THASRA, KATHLAL & KAPDWANJ TALUKAS OF NADIAD SSA ------ NOTE ------ Tender form can be down loaded from : 10:30 hours of 30/12/2015 To 17 : 00 hours Of 20/01/2016. Tender form to be submitted online : UP TO 14:00 Hrs. of 21/01/2016. Tender opening date & time [Online] : AT 14.30 Hrs. on 21/01/2016 Tender Form fee : Rs 500.00 (Non-refundable) NAME & ADDRESS OF THE TENDERER ______________________________ ______________________________ ______________________________ Phone (O): ___________________________ Mobile No: _________________________ ASSISTANT GENERAL MANAGER (CFA-PLG) O/O GENERAL MANEGER TELECOM DISTRICT 1st FLOOR, DOOR SANCHAR BHAVAN, PIJ ROAD TABLENADIAD OF -CONTENTS387 002 A.G.M. (CFA-PLG) O/o GMTD Nadiad Signature of Tenderer 2 INDEX Section contents Page No ______________________________________________________________________ Part – A Technical Bid 01 – 38 _________________________________________________________________________ I NIT 03-04 II BID Form 05 III Tenderer’s Profile 06 IV Eligibility & Documents required 07 V General Terms & Conditions of Tender 08-15 VI Scope Of Work 16 VII Special -

72/922 GJS NOVEMBER 2020 # Standwithstan on the Night of 8Th
72/922 GJS NOVEMBER 2020 PROVINCIAL’S PROGRAMME PROVINCE HIGHLIGHTS November # StandWithStan 03 - 05 : Visitation, Unai On the night of 8th October 09 : Meeting, Priests Council, Archdiocese of 2020, the 83-year-old Jesuit Gandhinagar priest, Fr Stan Swamy, was 18 - 21 : Superiors’ Forum taken into custody from his 21 - 22 : Consult residence in Bagaicha, Ranchi, by the National Intelligence 25 : Meeting, Adilok Agency (NIA) under the draconian UAPA (Unlawful 26 - 28 : Visitation, Surat Activities [Prevention] Act). He is now lodged in the 30 : Meeting, Commission for Higher Education Taloja Jail, near Mumbai. Several efforts are being made from all quarters to secure his immediate and unconditional release, and that of others incarcerated APPOINTMENTS under the UAPA. GENERAL has appointed Fr. Durai Fernand, our Provincial appointed a • Fr. Agnelo Mascarenhas SJ (GOA): Provincial of team consisting of Frs. Isaac Rumao (Convener), Pune Province Manoj Parmar, Robert Mascarenhas, Moieson Dhas, Cedric Prakash and Arul Rayan to coordinate • Fr. Fabien Gasigwa SJ: Regional Superior, Rwanda- the activities and response of the Province - to Burundi Region stand in solidarity with Fr. Stan. The team decided • Fr. José de Pablo SJ (ESP): Vice Ecclesiastical that Sunday 18th October (Mission Sunday) would Assistant, The World Christian Life Community be observed as a day of prayer for and solidarity (CLC) with Fr. Stan. Accordingly, Fr. Provincial wrote a letter to the Province and to the Bishops of Gujarat PROVINCIAL has appointed requesting all to observe the day. The coordinating • Fr. Joseph Mattam SJ: Assistant Parish Priest, team prepared appropriate posters, a prayer service, Anklav • Fr. Cyprian Monis SJ: Assistant Parish Priest, Bawla • Fr. -

District Census Handbook, 12 Kaira
CENSUS 1961 GUJARAT DISTRICT CENSUS HANDBOOK 12 K·,AIRA DISTRICT R. K. TRIVEDI Superintendent of Census ,Operations. Gujarat PRICE Rs. 9.95 of. DISTRICT: KAIRA l () '<'0 ~ '<'15' '0 --;:::--- i(/~ ,,' 1<1 ,0 \ a: , I ...,<f "-,.\ :;) ) :I.:l CENSUS OF INDIA 1961 LIST OF PUBLICATIONS CENTRAL GOVERNMENT PUBLICATIONS Census of India, 1961 Volume V-Gujarat is being published in the following parts: I-A General Report I--B Report on Vital S~atistics and Fertility Survey I-C Subsidiary Tables lI-A General Population Tables II-B(l) General Economic Tables (Tables B-1 to B-IV-C) I1-B(2) General Economic Tables (Tables B-V to B-IX) II-C Cultural and Migration Tables 111 Household Economic Tables (Tables B-X to B-XVII) IV-A Report on Housing and Establishments IV-B Housing and Establishment Tables V-A Tables on Scheduled Castes and Scheduled Tribes V-B Ethnographic Notes on Scheduled Castes and Scheduled Tribes (including reprints) VI Village Survey Monographs (25 Monographs) VII-A Selected Crafts of Gujarat VII-B Fairs and Festivals VIII-A Administra tion Report-Enumera tion I Not for Sale VIII-B Administration Report-Tabulation IX A tlas Volume X Special Report on Cities STATE GOVERNMENT PUBLICATIONS 17 District Census Handbooks in English 17 District Census Handbooks in Gujarati CONTENTS PREFACE ALPHABETICAL LIST OF VILLAGES PART I (i) rntroductory Essay . 1-35 (1) Location and Physical Features, (2) Administrative Set-up, (3) Local Self Government, (4) Population, (5) Housing, (6) Agriculture, (7) Livestock, (8) Irrigation, (9) Co-operation, -
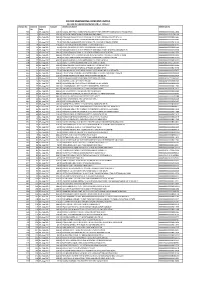
Elecon Engineering Company Limited Details of Unpaid Dividend for F.Y
ELECON ENGINEERING COMPANY LIMITED DETAILS OF UNPAID DIVIDEND FOR F.Y. 2016-17 Cheque No Warrant Warrant Amount Beneficiary Name Reference No No Date 580 1 05-Aug-2017 120.00 KAWAL JEET GILL C O BRIG SPS GILL DEPUTY GEN OFFICER COMMANDING HQ 24 INFAN 0000000000000K011604 581 2 05-Aug-2017 150.00 JAI SINGH RAWAT 4488 PUNJAB AND SIND BANK 00001201910101709134 585 6 05-Aug-2017 480.00 KAMLESH JAGGI C O SHRI T N JAGGI B 471 NEW FRIENDS COLONY MATHU 0000000000000K011985 588 9 05-Aug-2017 120.00 SATYAKAM C O RITES 27 BARAKHAMBA ROAD ROOM NO 605 NEW DELHI HOUSE 0000000000000S013492 590 11 05-Aug-2017 75.00 PRANOB CHAKRABORTY EMTICI ENG LTD 418 WORLD TRADE CENTRE 0000000000000P010194 593 14 05-Aug-2017 120.00 VEENA AHUJA 18 KHAN MARKET FLATS NEW DELHI 0000000000000V010147 594 15 05-Aug-2017 25.00 RAMA CHANDRA ACHARYA 233 JOR BAGH NEW DELHI 0000000000000R001546 595 16 05-Aug-2017 120.00 SANSAR CHAND SUD 1815 SECOND FLOOR UDAI CHAND M KOTLA MUBARAKPUR 0000000000000S013338 598 19 05-Aug-2017 160.00 PARSHOTAM LAL BAJAJ 1C 29 NEW ROHTAK ROAD NEW DELHI 0000000000000P011250 603 24 05-Aug-2017 480.00 VAIKUNTH NATH SHARMA 2 1786 NEAR BHAGIRATH PALACE CHANDNI CHOWK 0000000000000V011321 605 26 05-Aug-2017 80.00 MUKESH KAPOOR 506 KRISHNA GALI KATRA NEEL CHANDNI CHOWK 0000000000000M012161 606 27 05-Aug-2017 600.00 MOHD ASHEQIN C O GULAB TRADING CO 5585 S B DELHI 0000000000000M012009 608 29 05-Aug-2017 62.50 SHASHI CHOPRA 01190005348 STATE BANK OF INDIA 0000IN30039415130730 609 30 05-Aug-2017 120.00 USHA MATHUR 144 PRINCESS PARK PLOT NO 33 SECTOR 6 0000000000000U010436 -
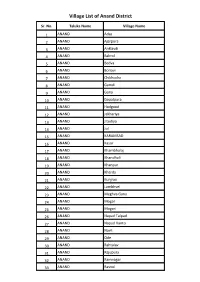
Village List of Anand District
Village List of Anand District Sr. No. Taluka Name Village Name 1 ANAND Adas 2 ANAND Ajarpura 3 ANAND Anklavdi 4 ANAND Bakrol 5 ANAND Bedva 6 ANAND Boriavi 7 ANAND Chikhodra 8 ANAND Gamdi 9 ANAND Gana 10 ANAND Gopalpura 11 ANAND Hadgood 12 ANAND Jakhariya 13 ANAND Jitodiya 14 ANAND Jol 15 ANAND KARAMSAD 16 ANAND Kasor 17 ANAND Khambholaj 18 ANAND Khandhali 19 ANAND Khanpur 20 ANAND Kherda 21 ANAND Kunjrao 22 ANAND Lambhvel 23 ANAND Meghva Gana 24 ANAND Mogar 25 ANAND Mogari 26 ANAND Napad Talpad 27 ANAND Napad Vanto 28 ANAND Navli 29 ANAND Ode 30 ANAND Rahtalav 31 ANAND Rajupura 32 ANAND Ramnagar 33 ANAND Rasnol 34 ANAND Samarkha 35 ANAND Sandesar 36 ANAND Sarsa 37 ANAND Sundan 38 ANAND Tarnol 39 ANAND Vadod 40 ANAND Vaghasi 41 ANAND Vaherakhadi 42 ANAND Valasan 43 ANAND Vans Khiliya 44 ANAND Vasad Village List of Petlad Taluka Sr. No. Taluka Name Village Name 1 PETLAD Agas 2 PETLAD Amod 3 PETLAD Ardi 4 PETLAD Ashi 5 PETLAD Bamroli 6 PETLAD Bandhani 7 PETLAD Bhalel 8 PETLAD Bhatiel 9 PETLAD Bhavanipura 10 PETLAD Bhurakui 11 PETLAD Boriya 12 PETLAD Changa 13 PETLAD Dantali 14 PETLAD Danteli 15 PETLAD Davalpura 16 PETLAD Demol 17 PETLAD Dhairyapura 18 PETLAD Dharmaj 19 PETLAD Fangani 20 PETLAD Ghunteli 21 PETLAD Isarama 22 PETLAD Jesarva 23 PETLAD Jogan 24 PETLAD Kaniya 25 PETLAD Khadana 26 PETLAD Lakkadpura 27 PETLAD Mahelav 28 PETLAD Manej 29 PETLAD Manpura 30 PETLAD Morad 31 PETLAD Nar 32 PETLAD Padgol 33 PETLAD Palaj 34 PETLAD Pandoli 35 PETLAD Petlad 36 PETLAD Porda 37 PETLAD Ramodadi 38 PETLAD Rangaipura 39 PETLAD Ravipura 40 PETLAD Ravli 41 PETLAD Rupiyapura 42 PETLAD Sanjaya 43 PETLAD Sansej 44 PETLAD Shahpur 45 PETLAD Shekhadi 46 PETLAD Sihol 47 PETLAD Silvai 48 PETLAD Simarada 49 PETLAD Sunav 50 PETLAD Sundara 51 PETLAD Sundarana 52 PETLAD Vadadala 53 PETLAD Vatav 54 PETLAD Virol(Simarada) 55 PETLAD Vishnoli 56 PETLAD Vishrampura Village List of Borsad Taluka Sr. -
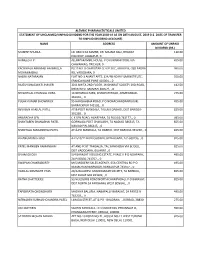
Name Address Amount of Unpaid Dividend (Rs.) Mukesh Shukla Lic Cbo‐3 Ka Samne, Dr
ALEMBIC PHARMACEUTICALS LIMITED STATEMENT OF UNCLAIMED/UNPAID DIVIDEND FOR THE YEAR 2018‐19 AS ON 28TH AUGUST, 2019 (I.E. DATE OF TRANSFER TO UNPAID DIVIDEND ACCOUNT) NAME ADDRESS AMOUNT OF UNPAID DIVIDEND (RS.) MUKESH SHUKLA LIC CBO‐3 KA SAMNE, DR. MAJAM GALI, BHAGAT 110.00 COLONEY, JABALPUR, 0 HAMEED A P . ALUMPARAMBIL HOUSE, P O KURANHIYOOR, VIA 495.00 CHAVAKKAD, TRICHUR, 0 KACHWALA ABBASALI HAJIMULLA PLOT NO. 8 CHAROTAR CO OP SOC, GROUP B, OLD PADRA 990.00 MOHMMADALI RD, VADODARA, 0 NALINI NATARAJAN FLAT NO‐1 ANANT APTS, 124/4B NEAR FILM INSTITUTE, 550.00 ERANDAWANE PUNE 410004, , 0 RAJESH BHAGWATI JHAVERI 30 B AMITA 2ND FLOOR, JAYBHARAT SOCIETY 3RD ROAD, 412.50 KHAR WEST MUMBAI 400521, , 0 SEVANTILAL CHUNILAL VORA 14 NIHARIKA PARK, KHANPUR ROAD, AHMEDABAD‐ 275.00 381001, , 0 PULAK KUMAR BHOWMICK 95 HARISHABHA ROAD, P O NONACHANDANPUKUR, 495.00 BARRACKPUR 743102, , 0 REVABEN HARILAL PATEL AT & POST MANDALA, TALUKA DABHOI, DIST BARODA‐ 825.00 391230, , 0 ANURADHA SEN C K SEN ROAD, AGARPARA, 24 PGS (N) 743177, , 0 495.00 SHANTABEN SHANABHAI PATEL GORWAGA POST CHAKLASHI, TA NADIAD 386315, TA 825.00 NADIAD PIN‐386315, , 0 SHANTILAL MAGANBHAI PATEL AT & PO MANDALA, TA DABHOI, DIST BARODA‐391230, , 0 825.00 B HANUMANTH RAO 4‐2‐510/11 BADI CHOWDI, HYDERABAD, A P‐500195, , 0 825.00 PATEL MANIBEN RAMANBHAI AT AND POST TANDALJA, TAL.SANKHEDA VIA BODELI, 825.00 DIST VADODARA, GUJARAT., 0 SIVAM GHOSH 5/4 BARASAT HOUSING ESTATE, PHASE‐II P O NOAPARA, 495.00 24‐PAGS(N) 743707, , 0 SWAPAN CHAKRABORTY M/S MODERN SALES AGENCY, 65A CENTRAL RD P O 495.00 -

CROCS of CHAROTAR Status, Distribution and Conservation of Mugger Crocodiles in Charotar, Gujarat, India
CROCS OF CHAROTAR Status, Distribution and Conservation of Mugger Crocodiles in Charotar, Gujarat, India THE DULEEP MATTHAI NATURE CONSERVATION TRUST Voluntary Nature Conservancy (VNC) acknowledges the support to this publication given by Ruff ord Small Grant Foundation, Duleep Matthai Nature Conservation Trust and Idea Wild. Published by Voluntary Nature Conservancy 101-Radha Darshan, Behind Union Bank, Vallabh Vidyanagar-388120, Gujarat, India ([email protected]) Designed by Niyati Patel & Anirudh Vasava Credits Report lead: Anirudh Vasava, Dhaval Patel, Raju Vyas Field work: Vishal Mistry, Mehul Patel, Kaushal Patel, Anirudh Vasava Data analysis: Anirudh Vasava, Niyati Patel Report Preparation: Anirudh Vasava Administrative support: Dhaval Patel Cover Photo: Mehul B. Patel Suggested Citation: Vasava A., Patel D., Vyas R., Mis- try V. & Patel M. (2015) Crocs of Charotar: Status, distri- bution and conservation of Mugger crocodiles in Charotar region , Gujarat, India. Voluntary Nature Conservancy, Vallabh Vidyanagar, India. Reproduction and dissemination of material in this pub- lication for educational or any non-commercial purpos- es are authorized without any prior written permission from the publisher provided the source is fully acknowl- edged and appropriate credit given. Reproduction of material in this information product for or other com- mercial purposes is prohibited without written permis- sion of the Publisher. Applications for such permission should be addressed to the Managing Trustee, Voluntary Nature Conservancy or by -

24 X 7 Primary Health Center
24 X 7 Primary Health Center Sr. No. District Name Taluka Name Sr. No. Name of PHC Dascroi 1 Kasindra Dascroi 2 Kuha Dascroi 3 Jetalpur Dascroi 4 Nandej Dascroi 5 Vehlal Sanand 6 Sanathal Sanand 7 Zolapur Sanand 8 Modasar Bavla 9 Nanodara Viramgam 10 Mandal 1 Ahmedabad Viramgam 11 Manipura Viramgam 12 Karakthal Viramgam 13 Kumarkhan Viramgam 14 Trent Dholka 15 Koth Dholka 16 Vataman Dholka 17 Transad Dhandhuka 18 Bhimnath Dhandhuka 19 Vagad Dhandhuka 20 Dholera Dhandhuka 21 Jalila Anand 22 Ajarpura Anand 23 Bakrol Anand 24 Karamsad Anand 25 Navali Anand 26 Vadod Anand 27 Vasad Anklav 28 Khadol Borsad 29 Badalpur Borsad 30 Davol Borsad 31 Napa 2 Anand Borsad 32 Sisva Borsad 33 Virsad Borsad 34 Zarola Khambhat 35 Undel Petlad 36 Bandhani Petlad 37 Changa Petlad 38 Devatalpad Petlad 39 Nar Umreth 40 Bhalej Umreth 41 Pansora 24 X 7 Primary Health Center Sr. No. District Name Taluka Name Sr. No. Name of PHC Nadiad 42 Palana Nadiad 43 Chaklasi Nadiad 44 Salun Nadiad 45 Maholel Nadiad 46 Yoginagar Nadiad 47 Pij Mahudha 48 Alina Kathalal 49 Gogjipura 3 Kheda Kathalal 50 Lasundra Kapadwanj 51 Antroli Matar 52 Alindra Matra 53 Limbasi Matar 54 Traj Kheda 55 Radhu Mahemdavad 56 Modaj Mahemdavad 57 Kanij Thasra 58 Sevaliya Patdi 59 Kherva Patdi 60 Dasada Wadhwan 61 Rampara Wadhwan 62 Dedadara Wadhwan 63 Vana Sayla 64 Doliya Sayla 65 Sudamda 4 Surendranagar Sayla 66 Tikar-Par Chotila 67 Bamanbore Limbdi 68 Panshina Limbdi 69 Mojidad Limbdi 70 Ranagadh Halvad 71 Tikar-Ran Dhrangadhra 72 Methan Dhrangadhra 73 Kondh 74 Pundra Mansa 75 Itadara 76 Rancharad Kalol 77 Pansar 78 Adalaj 5 Gandhinagar 79 Adaraj Gandhinagar 80 Uvarsad 81 Dabhoda 82 Bahiyal Dahegam 83 Sanoda 24 X 7 Primary Health Center Sr.