A Survey of Invasive Exotic Ants Found on Hawaiian Islands: Spatial Distributions and Patterns of Association
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ants in French Polynesia and the Pacific: Species Distributions and Conservation Concerns
Ants in French Polynesia and the Pacific: species distributions and conservation concerns Paul Krushelnycky Dept of Plant and Environmental Protection Sciences, University of Hawaii, Honolulu, Hawaii Hervé Jourdan Centre de Biologie et de Gestion des Populations, INRA/IRD, Nouméa, New Caledonia The importance of ants • In most ecosystems, form a substantial portion of a communities’ biomass (1/3 of animal biomass and ¾ of insect biomass in Amazon rainforest) Photos © Alex Wild The importance of ants • In most ecosystems, form a substantial portion of a communities’ biomass (1/3 of animal biomass and ¾ of insect biomass in Amazon rainforest) • Involved in many important ecosystem processes: predator/prey relationships herbivory seed dispersal soil turning mutualisms Photos © Alex Wild The importance of ants • Important in shaping evolution of biotic communities and ecosystems Photos © Alex Wild Ants in the Pacific • Pacific archipelagoes the most remote in the world • Implications for understanding ant biogeography (patterns of dispersal, species/area relationships, community assembly) • Evolution of faunas with depauperate ant communities • Consequent effects of ant introductions Hypoponera zwaluwenburgi Ants in the Amblyopone zwaluwenburgi Pacific – current picture Ponera bableti Indigenous ants in the Pacific? Approx. 30 - 37 species have been labeled “wide-ranging Pacific natives”: Adelomyrmex hirsutus Ponera incerta Anochetus graeffei Ponera loi Camponotus chloroticus Ponera swezeyi Camponotus navigator Ponera tenuis Camponotus rufifrons -

Download Article (PDF)
ISSN 0375-1511 Rec. zool. Surv. India: 113(Part-1): 169-182,2013 ON SOME ANTS (INSECTA: HYMENOPTERA: FORMICIDAE) FROM NAG ALAND, INDIA NEENA T AK AND SARFRAZUL ISLAM KAZMI Zoological Survey of India, New Alipore, Kolkata-700053, West Bengal, India INTRODUCTION organized and integrated units the societies or Nagaland is the hilly state of northeastern colonies. India having an area of 16,579 km2 lies between Bingham's (1903) fauna is the main source of 25°6/ and 274/ latitude, north of equator and knowledge of ants in India. Chapman and Capco between the longitudinal lines 93°20/Eand 95°15/E (1951) published a checklist of the ants of Asia. latitude, bounded by Tirap district of Arunachal Bolton (1995) has dealt with taxonomic and Pradesh in north-east, Assam in the west and Zoogeographical census of the extant ant taxa northwest, Manipur in the south while the (Hymenoptera: Formicidae). Bolton has eastern limits are continuous with the published a Catalogue of Ants of the World CD international boundary between India and ROM 2007(1758-2005). Datta & Raychaudhuri Myanmar. This is predominantly a hilly state (1985) has reported a new species of ant with valleys, streams and mountains. Nagaland (Hymenoptera : Formicidae) from Nagaland, includes the former Naga hill district of Assam north-east India in Science and Culture. In the (established in 1881) and the Tuensang division of State Fauna Series (Fauna of Nagaland, 2005) 5 North East Frontier Agency (NEFA, now Orders namely Orthoptera, Lepidoptera, Arunachal Pradesh). The administrative "unit" Odonata, Diptera and Coleoptera of Class Insecta known as the "Naga hills" and tuensang Area" has been worked out, but Order Hymenoptera (NHTA) was established on r t December 1957. -

A Guide to the Ants of Sabangau
A Guide to the Ants of Sabangau The Orangutan Tropical Peatland Project November 2014 A Guide to the Ants of Sabangau All original text, layout and illustrations are by Stijn Schreven (e-mail: [email protected]), supple- mented by quotations (with permission) from taxonomic revisions or monographs by Donat Agosti, Barry Bolton, Wolfgang Dorow, Katsuyuki Eguchi, Shingo Hosoishi, John LaPolla, Bernhard Seifert and Philip Ward. The guide was edited by Mark Harrison and Nicholas Marchant. All microscopic photography is from Antbase.net and AntWeb.org, with additional images from Andrew Walmsley Photography, Erik Frank, Stijn Schreven and Thea Powell. The project was devised by Mark Harrison and Eric Perlett, developed by Eric Perlett, and coordinated in the field by Nicholas Marchant. Sample identification, taxonomic research and fieldwork was by Stijn Schreven, Eric Perlett, Benjamin Jarrett, Fransiskus Agus Harsanto, Ari Purwanto and Abdul Azis. Front cover photo: Workers of Polyrhachis (Myrma) sp., photographer: Erik Frank/ OuTrop. Back cover photo: Sabangau forest, photographer: Stijn Schreven/ OuTrop. © 2014, The Orangutan Tropical Peatland Project. All rights reserved. Email [email protected] Website www.outrop.com Citation: Schreven SJJ, Perlett E, Jarrett BJM, Harsanto FA, Purwanto A, Azis A, Marchant NC, Harrison ME (2014). A Guide to the Ants of Sabangau. The Orangutan Tropical Peatland Project, Palangka Raya, Indonesia. The views expressed in this report are those of the authors and do not necessarily represent those of OuTrop’s partners or sponsors. The Orangutan Tropical Peatland Project is registered in the UK as a non-profit organisation (Company No. 06761511) and is supported by the Orangutan Tropical Peatland Trust (UK Registered Charity No. -

Taxonomic Studies on Ant Genus Hypoponera (Hymenoptera: Formicidae: Ponerinae) from India
ASIAN MYRMECOLOGY Volume 7, 37 – 51, 2015 ISSN 1985-1944 © HIMENDER BHARTI, SHAHID ALI AKBAR, AIJAZ AHMAD WACHKOO AND JOGINDER SINGH Taxonomic studies on ant genus Hypoponera (Hymenoptera: Formicidae: Ponerinae) from India HIMENDER BHARTI*, SHAHID ALI AKBAR, AIJAZ AHMAD WACHKOO AND JOGINDER SINGH Department of Zoology and Environmental Sciences, Punjabi University, Patiala – 147002, India *Corresponding author's e-mail: [email protected] ABSTRACT. The Indian species of the ant genus Hypoponera Santschi, 1938 are treated herewith. Eight species are recognized of which three are described as new and two infraspecific taxa are raised to species level. The eight Indian species are: H. aitkenii (Forel, 1900) stat. nov., H. assmuthi (Forel, 1905), H. confinis (Roger, 1860), H. kashmirensis sp. nov., H. shattucki sp. nov., H. ragusai (Emery, 1894), H. schmidti sp. nov. and H. wroughtonii (Forel, 1900) stat. nov. An identification key based on the worker caste of Indian species is provided. Keywords: New species, ants, Formicidae, Ponerinae, Hypoponera, India. INTRODUCTION genus with use of new taxonomic characters facilitating prompt identification. The taxonomy of Hypoponera has been in a From India, three species and two state of confusion and uncertainty for some infraspecific taxa ofHypoponera have been reported time. The small size of the ants, coupled with the to date (Bharti, 2011): Hypoponera assmuthi morphological monotony has led to the neglect (Forel, 1905), Hypoponera confinis (Roger, of this genus. The only noteworthy revisionary 1860), Hypoponera confinis aitkenii (Forel, 1900), work is that of Bolton and Fisher (2011) for Hypoponera confinis wroughtonii (Forel, 1900) and the Afrotropical and West Palearctic regions. Hypoponera ragusai (Emery, 1894). -

Том 16. Вып. 2 Vol. 16. No. 2
РОССИЙСКАЯ АКАДЕМИЯ НАУК Южный научный центр RUSSIAN ACADEMY OF SCIENCES Southern Scientific Centre CAUCASIAN ENTOMOLOGICAL BULLETIN Том 16. Вып. 2 Vol. 16. No. 2 Ростов-на-Дону 2020 Кавказский энтомологический бюллетень 16(2): 381–389 © Caucasian Entomological Bulletin 2020 Contribution of wet zone coconut plantations and non-agricultural lands to the conservation of ant communities (Hymenoptera: Formicidae) in Sri Lanka © R.K.S. Dias, W.P.S.P. Premadasa Department of Zoology and Environmental Management, Faculty of Science, University of Kelaniya, Kelaniya 11600 Sri Lanka. E-mails: [email protected], [email protected] Abstract. Agricultural practices are blamed for the reduction of ant diversity on earth. Contribution of four coconut plantations (CP) and four non-agricultural lands (NL) for sustaining diversity and relative abundance of ground-dwelling and ground-foraging ants was investigated by surveying them from May to October, 2018, in a CP and a NL in Minuwangoda, Mirigama, Katana and Veyangoda in Gampaha District that lies in the wet zone, Sri Lanka. Worker ants were surveyed by honey baiting and soil sifting along two transects at three, 50 m2 plots in each type of land. Workers were identified using standard methods and frequency of each ant species observed by each method was recorded. Percentage frequency of occurrence observed by each method, mean percentage frequency of occurrence of each ant species and proportional abundance of each species in each ant community were calculated. Species richness recorded by both methods at each CP was 14–19 whereas that recorded at each NL was 17–23. Shannon-Wiener Diversity Index values (Hʹ, CP: 2.06–2.36; NL: 2.11–2.56) and Shannon- Wiener Equitability Index values (Jʹ, CP: 0.73–0.87; NL: 0.7–0.88) showed a considerable diversity and evenness of ant communities at both types of lands. -

Ants (Hymenoptera: Fonnicidae) of Samoa!
Ants (Hymenoptera: Fonnicidae) of Samoa! James K Wetterer 2 and Donald L. Vargo 3 Abstract: The ants of Samoa have been well studied compared with those of other Pacific island groups. Using Wilson and Taylor's (1967) specimen records and taxonomic analyses and Wilson and Hunt's (1967) list of 61 ant species with reliable records from Samoa as a starting point, we added published, unpublished, and new records ofants collected in Samoa and updated taxonomy. We increased the list of ants from Samoa to 68 species. Of these 68 ant species, 12 species are known only from Samoa or from Samoa and one neighboring island group, 30 species appear to be broader-ranged Pacific natives, and 26 appear to be exotic to the Pacific region. The seven-species increase in the Samoan ant list resulted from the split of Pacific Tetramorium guineense into the exotic T. bicarinatum and the native T. insolens, new records of four exotic species (Cardiocondyla obscurior, Hypoponera opaciceps, Solenopsis geminata, and Tetramorium lanuginosum), and new records of two species of uncertain status (Tetramorium cf. grassii, tentatively considered a native Pacific species, and Monomorium sp., tentatively considered an endemic Samoan form). SAMOA IS AN ISLAND CHAIN in western island groups, prompting Wilson and Taylor Polynesia with nine inhabited islands and (1967 :4) to feel "confident that a nearly numerous smaller, uninhabited islands. The complete faunal list could be made for the western four inhabited islands, Savai'i, Apo Samoan Islands." Samoa is of particular in lima, Manono, and 'Upolu, are part of the terest because it is one of the easternmost independent country of Samoa (formerly Pacific island groups with a substantial en Western Samoa). -
Plates for Ants of Micronesia.Pdf
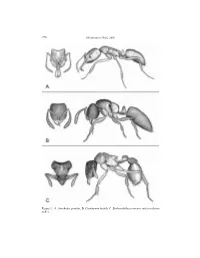
276 Micronesica 39(2), 2007 Figure 1. A–Anochetus graeffei; B–Cryptopone butteli; C–Eurhopalothrix procera (not to relative scale). Clouse: Ants of Micronesia 277 Figure 2. Camponotus mesosoma lateral profiles (not to scale): A– sp. 121958, B–chloroticus, C– eperiamorum, D–erythrocephalus, E–marianensis, F–reticulatus. 278 Micronesica 39(2), 2007 Figure 3. A–Cardiocondyla obscurior lateral, B–Cerapachys 91952 lateral anterior, C–lateral posterior, D–Crematogaster biroi / emeryi propodeal spine; E–Crematogaster fritzi propodeal spine; F–Cryptopone butteli head side. Clouse: Ants of Micronesia 279 Figure 4. A–Cryptopone butteli node lateral; B– C. testacea node lateral; C–Hypoponera confinis head lateral; D–H. punctatissima node lateral; E–Monomorium chinense-group petiole and postpetiole lateral; F–M. floricola petiole and postpetiole lateral 280 Micronesica 39(2), 2007 Figure 5. A–Monomorium australicum lateral; B–M. sechellense lateral; C–Myrmecina sp. 7121952 head side, D–front; E–Paratrechina bourbonica lateral mesonotum; F–P. vaga lateral mesonotum. Clouse: Ants of Micronesia 281 Figure 6. A–Paratrechina clandestina lateral, B–front, C–propodeum, D–nozzle. 282 Micronesica 39(2), 2007 Figure 7. A–Pheidole sp. 24041958 lateral, B–front; C–P. fervens major front close-up, D–minor propodeum; E–P. oceanica major front close-up, F–minor propodeum. Clouse: Ants of Micronesia 283 Figure 8. A–Pheidole megacephala propodeum; B–Pheidole recondita minor; C–Platythyrea parallela tarsal claw; D–Polyrachis sp. 91952; E–Ponera sp. 10091995 dorsal; F–P. tenuis dorsal. 284 Micronesica 39(2), 2007 Figure 9. A–Ponera tenuis; B–Prionopelta opaca (not to relative scale). Clouse: Ants of Micronesia 285 Figure 10. -

Invasive Ant Pest Risk Assessment Project: Preliminary Risk Assessment
Invasive ant pest risk assessment project: Preliminary risk assessment Harris, R. 1) Aim To assess the threat to New Zealand of a wide range of ant species not already established in New Zealand and identify those worthy of more detailed assessment. 2) Scope 2.1. Specific exclusions Solenopsis invicta was specifically excluded from consideration as this species has already been subject to detailed consideration by Biosecurity New Zealand. 2.2 Specific inclusions Biosecurity New Zealand requested originally that the following taxa be included in the assessment: Solenopsis richteri Solenopsis geminata Wasmannia auropunctata Anoplolepis gracilipes Paratrechina longicornis Carpenter ants (Camponotus spp.) Leaf cutting ants (Atta spp.) Myrmecia pilosula Tapinoma melanocephalum Monomorium sydneyense (incursion found in New Zealand) Hypoponera punctatissima (incursion found in New Zealand) Big headed ants (Pheidole spp.) M. sydneyense and H. punctatissima have since been deemed not under official control and are now considered established in New Zealand. Profiles of these species have been prepared as part of the Ants of New Zealand section (see http://www.landcareresearch.co.nz/research/biosecurity/stowaways/Ants/antsinnewzealand.asp). INVASIVE ANT PEST RISK ASSESSMENT PROJECT: Preliminary risk assessment 3) Methodology A risk assessment scorecard was developed (Appendix 1) in consultation with a weed risk assessment expert (Dr Peter Williams) and with Simon O’Connor and Amelia Pascoe of Biosecurity New Zealand, to initially separate -

Ben Hoffmann CV
CURRICULUM VITAE - BEN HOFFMANN Personal details Name : Benjamin Daniel Hoffmann Date of Birth : 4th December 1975 Contact Details (work) (home) CSIRO Ecosystem Sciences PO Box 1682 PMB 44 Winnellie Humpty Doo NT 0822 NT 0835 Ph. +61 8 89448432 Ph. +61 8 8988 1315 Mobile +61 418 820 718 Email [email protected] Education Undergraduate Bachelor of Science (Bsc). 1993-1995, Northern Territory University, Darwin Bsc. (Honours). 1996 , Northern Territory University, Darwin Honours Project Title - Ecology of the introduced ant Pheidole megacephala in the Howard Springs region of Australia’s Northern Territory. Postgraduate PhD. 1997-2001 , Northern Territory University, Darwin Thesis Title - Responses of ant communities to land use impacts in Australia. Employment of Relevance 2004 – present CSIRO Darwin. Research of invasive ant biology, ecology, impacts and management. Coordinating exotic ant eradications. Member on scientific advisory panels providing advise to other ant management programs. Research into disturbance ecology particularly minesite rehabilitation, utilizing ants as biological indicators. 1998 – 2004 CSIRO Darwin, Numerous small consultancies, particularly minesite rehabilitation assessments and sorting ants for other researchers. Journal articles (51) Hoffmann BD , Courchamp F (in review) Biological invasions and natural colonisations: are they different? Trends in Ecology and Evolution Hoffmann BD , Broadhurst LM (in review) The economic cost of invasive species to Australia. BioScience Gibb H, Sanders NJ, Dunn RR, Photakis M, Abril S, Andersen AN, Angulo E, Armbrecht I, Arnan, X, Baccaro FB, Boulay R, Castracani C, Del Toro I, Delsinne T, Diaz M, Donoso DA, Enríquez ML, Fayle TM, Feener Jr DH, Fitzpatrick M, Gómez C, Grasso DA, Groc S, Heterick B, Hoffmann BD , Lach L, Lattke J, Leponce M, Lessard JP, Longino J, Lucky A, Majer J, Menke SB, Mezger D, Mori A, Nia OP, Perace-Duvet J, Pfeiffer M, Philpott S, de Souza JLP, Tista M, Vonshak M, Parr CL (in review) Climate regulates the effects of anthropogenic disturbance on ant assemblage structure. -

Hymenoptera: Formicidae
16 The Weta 30: 16-18 (2005) Changes to the classification of ants (Hymenoptera: Formicidae) Darren F. Ward School of Biological Sciences, Tamaki Campus, Auckland University, Private Bag 92019, Auckland ([email protected]) Introduction This short note aims to update the reader on changes to the subfamily classification of ants (Hymenoptera: Formicidae). Although the New Zealand ant fauna is very small, these changes affect the classification and phylogeny of both endemic and exotic ant species in New Zealand. Bolton (2003) has recently proposed a new subfamily classification for ants. Two new subfamilies have been created, a revised status for one, and new status for four. Worldwide, there are now 21 extant subfamilies of ants. The endemic fauna of New Zealand is now classified into six subfamilies (Table 1), as a result of three subfamilies, Amblyoponinae, Heteroponerinae and Proceratiinae, being split from the traditional subfamily Ponerinae. Bolton’s (2003) classification also affects several exotic species in New Zealand. Three species have been transferred from Ponerinae: Amblyopone australis to Amblyoponinae, and Rhytidoponera chalybaea and R. metallica to Ectatomminae. Currently there are 28 exotic species in New Zealand (Table 1). Eighteen species have most likely come from Australia, where they are native. Eight are global tramp species, commonly transported by human activities, and two species are of African origin. Nineteen of the currently established exotic species are recorded for the first time in New Zealand as occurring outside their native range. This may result in difficulty in obtaining species-specific biological knowledge and assessing their likelihood of becoming successful invaders. In addition to the work by Bolton (2003), Phil Ward and colleagues at UC Davis have started to resolve the phylogenetic relationships among subfamilies and genera of all ants using molecular data (Ward et al, 2005). -

36 Wood Destroying Insects
CHAPTER 36 THE BEST CONTROL OR HOW TO PERMANENTLY AND SAFELY CONTROL ALL WOOD DESTROYING ORGANISMS http://www.pctonline.com/copesan/ (without killing yourself) The February 1999 issue of Pest Control magazine on page 18 quotes Dr. Austin Frishman as saying, “We know that termiticides alone will not solve most termite problems.” This chapter will show you how to safely solve them without using any volatile termiticide poisons. At the time a live tree is cut down, nearly half its weight consists of water! The most destructive factor to wood in structures is excessive moisture, not wood destroying insects. Correct all moisture and humidity problems and you will also control almost all wood destroying insect problems without using any poisons. Use ventilation, moisture barriers, fans, air conditioners and/or dehumidifiers first, last and always. 1347 FORWARD Far more volatile, “registered,” synthetic pesticide poison is used to control termites than any other structural pest you will ever encounter. No volatile synthetic residual insecticide or economic poison is completely safe no matter what the professional pest control industry claims. The U. S. Environmental Protection Agency (EPA), when it approves one of the economic poisons, basically is only concerned with the harmful effects that occur from a single exposure of only the active ingredient by any route of entry or its acute toxicity expressed as its LD50 or LC50 value which is the lethal dose or concentration (relative amount) of only the active ingredient required to kill 50 % of a test population, e.g., male rats. LD50 values are recorded in milligrams of active ingredient per kilogram of body weight of the test animal. -

Bulletin of the British Museum (Natural History) Entomology
Bulletin of the British Museum (Natural History) A review of the Solenopsis genus-group and revision of Afrotropical Monomorium Mayr (Hymenoptera: Formicidae) Barry Bolton Entomology series Vol 54 No 3 25 June 1987 The Bulletin of the British Museum (Natural History), instituted in 1949, is issued in four scientific series, Botany, Entomology, Geology (incorporating Mineralogy) and Zoology, and an Historical series. Papers in the Bulletin are primarily the results of research carried out on the unique and ever-growing collections of the Museum, both by the scientific staff of the Museum and by specialists from elsewhere who make use of the Museum's resources. Many of the papers are works of reference that will remain indispensable for years to come. Parts are published at irregular intervals as they become ready, each is complete in itself, available separately, and individually priced. Volumes contain about 300 pages and several volumes may appear within a calendar year. Subscriptions may be placed for one or more of the series on either an Annual or Per Volume basis. Prices vary according to the contents of the individual parts. Orders and enquiries should be sent to: Publications Sales, British Museum (Natural History), Cromwell Road, London SW7 5BD, England. World List abbreviation: Bull. Br. Mus. nat. Hist. (Ent.) ©British Museum (Natural History), 1987 The Entomology series is produced under the general editorship of the Keeper of Entomology: Laurence A. Mound Assistant Editor: W. Gerald Tremewan ISBN 565 06026 ISSN 0524-6431 Entomology