Entropy Maximization in the Free Expansion Process
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamics of Ideal Gases
D Thermodynamics of ideal gases An ideal gas is a nice “laboratory” for understanding the thermodynamics of a fluid with a non-trivial equation of state. In this section we shall recapitulate the conventional thermodynamics of an ideal gas with constant heat capacity. For more extensive treatments, see for example [67, 66]. D.1 Internal energy In section 4.1 we analyzed Bernoulli’s model of a gas consisting of essentially 1 2 non-interacting point-like molecules, and found the pressure p = 3 ½ v where v is the root-mean-square average molecular speed. Using the ideal gas law (4-26) the total molecular kinetic energy contained in an amount M = ½V of the gas becomes, 1 3 3 Mv2 = pV = nRT ; (D-1) 2 2 2 where n = M=Mmol is the number of moles in the gas. The derivation in section 4.1 shows that the factor 3 stems from the three independent translational degrees of freedom available to point-like molecules. The above formula thus expresses 1 that in a mole of a gas there is an internal kinetic energy 2 RT associated with each translational degree of freedom of the point-like molecules. Whereas monatomic gases like argon have spherical molecules and thus only the three translational degrees of freedom, diatomic gases like nitrogen and oxy- Copyright °c 1998{2004, Benny Lautrup Revision 7.7, January 22, 2004 662 D. THERMODYNAMICS OF IDEAL GASES gen have stick-like molecules with two extra rotational degrees of freedom or- thogonally to the bridge connecting the atoms, and multiatomic gases like carbon dioxide and methane have the three extra rotational degrees of freedom. -

Chem 322, Physical Chemistry II: Lecture Notes
Chem 322, Physical Chemistry II: Lecture notes M. Kuno December 20, 2012 2 Contents 1 Preface 1 2 Revision history 3 3 Introduction 5 4 Relevant Reading in McQuarrie 9 5 Summary of common equations 11 6 Summary of common symbols 13 7 Units 15 8 Math interlude 21 9 Thermo definitions 35 10 Equation of state: Ideal gases 37 11 Equation of state: Real gases 47 12 Equation of state: Condensed phases 55 13 First law of thermodynamics 59 14 Heat capacities, internal energy and enthalpy 79 15 Thermochemistry 111 16 Entropy and the 2nd and 3rd Laws of Thermodynamics 123 i ii CONTENTS 17 Free energy (Helmholtz and Gibbs) 159 18 The fundamental equations of thermodynamics 179 19 Application of Free Energy to Phase Transitions 187 20 Mixtures 197 21 Equilibrium 223 22 Recap 257 23 Kinetics 263 Chapter 1 Preface What is the first law of thermodynamics? You do not talk about thermodynamics What is the second law of thermodynamics? You do not talk about thermodynamics 1 2 CHAPTER 1. PREFACE Chapter 2 Revision history 2/05: Original build 11/07: Corrected all typos caught to date and added some more material 1/08: Added more stuff and demos. Made the equilibrium section more pedagogical. 5/08: Caught more typos and made appropriate fixes to some nomenclature mistakes. 12/11: Started the next round of text changes and error corrections since I’m teaching this class again in spring 2012. Woah! I caught a whole bunch of problems that were present in the last version. It makes you wonder what I was thinking. -

Physical Chemistry I
Physical Chemistry I Andrew Rosen August 19, 2013 Contents 1 Thermodynamics 5 1.1 Thermodynamic Systems and Properties . .5 1.1.1 Systems vs. Surroundings . .5 1.1.2 Types of Walls . .5 1.1.3 Equilibrium . .5 1.1.4 Thermodynamic Properties . .5 1.2 Temperature . .6 1.3 The Mole . .6 1.4 Ideal Gases . .6 1.4.1 Boyle’s and Charles’ Laws . .6 1.4.2 Ideal Gas Equation . .7 1.5 Equations of State . .7 2 The First Law of Thermodynamics 7 2.1 Classical Mechanics . .7 2.2 P-V Work . .8 2.3 Heat and The First Law of Thermodynamics . .8 2.4 Enthalpy and Heat Capacity . .9 2.5 The Joule and Joule-Thomson Experiments . .9 2.6 The Perfect Gas . 10 2.7 How to Find Pressure-Volume Work . 10 2.7.1 Non-Ideal Gas (Van der Waals Gas) . 10 2.7.2 Ideal Gas . 10 2.7.3 Reversible Adiabatic Process in a Perfect Gas . 11 2.8 Summary of Calculating First Law Quantities . 11 2.8.1 Constant Pressure (Isobaric) Heating . 11 2.8.2 Constant Volume (Isochoric) Heating . 11 2.8.3 Reversible Isothermal Process in a Perfect Gas . 12 2.8.4 Reversible Adiabatic Process in a Perfect Gas . 12 2.8.5 Adiabatic Expansion of a Perfect Gas into a Vacuum . 12 2.8.6 Reversible Phase Change at Constant T and P ...................... 12 2.9 Molecular Modes of Energy Storage . 12 2.9.1 Degrees of Freedom . 12 1 2.9.2 Classical Mechanics . -

Thermodynamics
...Thermodynamics Clausius Inequality R dq Existence of a definite integral T Entropy - a state variable Second Law stated as principle of increasing entropy Entropy changes in system and the surroundings October 9, 2018 1 / 20 The Principle of Increasing Entropy Isolated system, or System plus Surroundings enclosed by an adiabatic wall, or entire universe. d¯q = 0 ) ∆S ≥ 0. The entropy of a thermally isolated ensemble can never decrease. This defines a direction of time. The Second Law is the ONLY fundamental equation in physics which violates CPT Symmetry. Boltzmann argued that its validity was not based on the inherent laws of nature but rather on the choice of extremely improbable initial conditions. October 9, 2018 2 / 20 The Central Equation of Thermodynamics Combine the first and second laws. dU = d¯Q + d¯W ! dU = TdS - PdV Derived for infinitesimal reversible processes in differential form. This equation involves only state variables. Can be integrated to relate changes of state variables independent of the path of integration. Integration of equation can be applied to irreversible processes. Can be written TdS = dU + PdV , relating entropy to more easily measurable quantities. U and V are extensive quantities, so S must also be extensive. October 9, 2018 3 / 20 Caveat: PdV implies mechanical work compressing a fluid. In general d¯W should include all forms of work. e.g. For electric charge (Z) and emf E, dU = TdS − PdV + EdZ. For a rubber band length L under tension F, dU = TdS + FdL. October 9, 2018 4 / 20 Entropy of an ideal gas For an Ideal gas U = U(T ), so dU = Cv dT . -
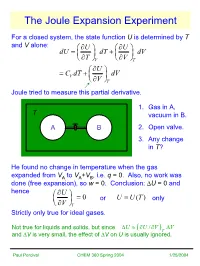
The Joule Expansion Experiment
The Joule Expansion Experiment For a closed system, the state function U is determined by T and V alone: ∂UU ∂ dU=+ dT dV ∂∂TVVT ∂U =+CdTV dV ∂V T Joule tried to measure this partial derivative. 1. Gas in A, T vacuum in B. AB2. Open valve. 3. Any change in T? He found no change in temperature when the gas expanded from VA to VA+VB, i.e. q = 0. Also, no work was done (free expansion), so w = 0. Conclusion: ∆U = 0 and hence ∂U = 0 orUUT= () only ∂V T Strictly only true for ideal gases. Not true for liquids and solids, but since ∆UUVV≈∂/ ∂ ∆ ( )T and ∆V is very small, the effect of ∆V on U is usually ignored. Paul Percival CHEM 360 Spring 2004 1/25/2004 Pressure Dependence of Enthalpy For a closed system, the state function H is determined by T and P alone: ∂HH ∂ dH=+ dT dP ∂∂TPPT ∂H =+CdTP dP ∂P T For ideal gases HUPVUnRT=+ =+ ∂∂HU = = 0 ∂∂PPTT Not true for real gases, liquids and solids! dH=+ dU PdV + VdP ∂∂HU CPV dT+=+++ dP C dT dV PdV VdP ∂∂PVTT At fixed temperature, dT = 0 ∂∂∂∂HUVV =++PV ≈ V ∂∂∂∂PVPPTTTT small for liquids and solids Investigate real gases at constant enthalpy, i.e. dH = 0 ∂∂HT ⇒ =−CP ∂∂PPTH Paul Percival CHEM 360 Spring 2004 1/25/2004 The Joule-Thomson Experiment ∂T Joule-Thomson Coefficient µ=JT ∂P H Pump gas through throttle (hole or porous plug) P1 > P2 P1 Keep pressures constant by moving pistons. Work done by system −=wPVPV22 − 11 Since q = 0 P2 ∆=UU21 − U == wPVPV 1122 − ⇒ UPVUPV222111+=+ HH21= constant enthalpy Measure change in T of gas as it moves from side 1 to side 2. -

Real Gas Expansions
Handout 11 Real gas expansions Joule expansion A Joule expansion is an irreversible expansion of a gas into an initially evacuated container with adiathermal walls. No work is done on the gas and no heat enters the gas, so ∆U = 0. The temperature change is described by the Joule coefficient, ! " ! # @T 1 @p µJ = = − T − p : (1) @V U CV @T V 2 For an ideal gas, µJ = 0. For a van der Waals gas, µJ = −a=(CV V ). Joule{Kelvin (Joule{Thomson) expansion In a Joule{Kelvin expansion, the gas is expanded adiathermally from pressure p1 to p2 by a steady flow through a porous plug (throttle valve). ∆Q = 0, so enthalpy is conserved: ∆U = ∆W ) U2 − U1 = p1V1 − p2V2 ) H1 = H2: (2) The temperature change is described by the Joule{Kelvin coefficient, ! 2 ! 3 @T 1 4 @V 5 µJK = = T − V : (3) @p H Cp @T p For a van der Waals gas, ( ) 1 2a lim µ = − b : (4) ! JK p 0 Cp RT This can be positive or negative, so both heating and cooling are possible. The diagram on the right shows the isenthalps of nitrogen in the p − T plane. The inversion curve is the line separating regions with µJK > 0 and µJK < 0. [From A. Kent, Experimental Low Temper- ature Physics (MacMillan, 1993).] 1 Liquefaction of gases Liquefaction by the Linde cycle. A Joule{Kelvin gas expansion from the region in the p − T diagram where µJK < 0 to the region where µJK > 0 can produce strong cooling. Gas at a high initial pressure p1 and tempera- ture T1 expands into a chamber at a much lower pressure pL ∼ 1 atm. -

Notes for Thermal Physics Course, CMI, Autumn 2019 Govind S. Krishnaswami, November 19, 2019 Contents 1
Notes for Thermal Physics course, CMI, Autumn 2019 Govind S. Krishnaswami, November 19, 2019 These lecture notes are very sketchy and are no substitute for books, attendance and taking notes at lectures. More information will be given on the course web site http://www.cmi.ac.in/~govind/teaching/thermo-o19. Please let me know (via [email protected]) of any comments or corrections. Help from Sonakshi Sachdev in preparing these notes is gratefully acknowledged. Contents 1 Thermodynamic systems and states 1 1.1 Thermodynamic state of a gas and a paramagnet..........................................2 1.2 Thermodynamic processes.......................................................4 1.3 Work..................................................................6 1.4 Exact and inexact differentials....................................................7 1.5 Ideal gas laws.............................................................9 2 Heat transferred and the first law of thermodynamics 12 2.1 Heat transferred and its mechanical equivalent............................................ 12 2.2 First Law of Thermodynamics.................................................... 13 2.3 First law for systems with ( p; V; T ) state space, three δQ equations and specific heats...................... 14 2.4 First law for paramagnet....................................................... 15 3 Applications of the first law 15 3.1 Gay-Lussac and Joule experiment: irreversible adiabatic expansion................................. 15 3.2 Joule-Kelvin (or Joule-Thomson) porous -

Action and Entropy in Heat Engines: an Action Revision of the Carnot Cycle
entropy Article Action and Entropy in Heat Engines: An Action Revision of the Carnot Cycle Ivan R. Kennedy 1,* and Migdat Hodzic 2 1 School of Life and Environmental Sciences, Sydney Institute of Agriculture, University of Sydney, Sydney, NSW 2006, Australia 2 Faculty of Information Technologies (FIT), University of Mostar, 88000 Mostar, Bosnia and Herzegovina; [email protected] * Correspondence: [email protected] Abstract: Despite the remarkable success of Carnot’s heat engine cycle in founding the discipline of thermodynamics two centuries ago, false viewpoints of his use of the caloric theory in the cycle linger, limiting his legacy. An action revision of the Carnot cycle can correct this, showing that the heat flow powering external mechanical work is compensated internally with configurational changes in the thermodynamic or Gibbs potential of the working fluid, differing in each stage of the cycle quantified by Carnot as caloric. Action (@) is a property of state having the same physical dimensions as angular momentum (mrv = mr2w). However, this property is scalar rather than vectorial, including a dimensionless phase angle (@ = mr2wdj). We have recently confirmed with atmospheric gases that their entropy is a logarithmic function of the relative vibrational, rotational, and translational action ratios with Planck’s quantum of action h¯ . The Carnot principle shows that the maximum rate of work (puissance motrice) possible from the reversible cycle is controlled by the difference in temperature of the hot source and the cold sink: the colder the better. This temperature difference between the source and the sink also controls the isothermal variations of the Gibbs potential of the Citation: Kennedy, I.R.; Hodzic, M. -

Midterm 1 Questions
Name: WWWWWWW Midterm 1 SID: WWWWWWW Midterm 1 Please fill in your name and student id There is a total of four sections in this exam: Section 1. True or false • Section 2. Fill in the blank • Section 3. Multiple choice • Section 4. Short-to-medium length questions • For Section 1-3, you are only graded based on your final answers. For Section 4, you will be graded based on your final answers and showing your work. The exam is closed book, closed notes, and no electronic devices List of Equations An exact di↵erential for a multi-variable function: @f @f @f df = dx + ...+ dx + ...+ dx @x 1 @x j @x m 1 xi=1 j xi=j m xi=m ✓ ◆ 6 ✓ ◆ 6 ✓ ◆ 6 Gibbs phase rule: F =2+N N c − ph Ideal gas’ molar internal energy and enthalpy (both for constant and temperature-dependent heat capacities): u = c (T ) T ; h = c (T ) T ig v · ig p · Total energy balance and the 2nd law in Open Systems: 1 1 0=Q˙ + W˙ + m˙ hˆ + V 2 + gz m˙ hˆ + V 2 + gz s 2 − 2 in out out Xin ✓ ◆ X ✓ ◆ dS Q˙ + (˙msˆ) (˙msˆ) 0 dt out − in − T ≥ sys out surr. ✓ ◆ X Xin Change of entropy in an ideal gas with temperature-independent heat capacity: T V T P P V γ ∆s = c ln 2 + R ln 2 = c ln 2 R ln 2 = c ln 2 2 v T V p T − P v P V 1 1 1 1 ✓ 1 ✓ 1 ◆ ◆ where γ = cp/cv. -

Lecture Notes on Thermodynamics
Lecture Notes on Thermodynamics Éric Brunet1, Thierry Hocquet2, Xavier Leyronas3 February 13, 2019 A theory is the more impressive the greater the simplicity of its premises is, the more different kinds of things it relates, and the more extended is its area of applicability. Therefore the deep impression which classical thermodynamics made upon me. It is the only physical theory of universal content concerning which I am convinced that, within the framework of the applicability of its basic concepts, it will never be overthrown. Albert Einstein, 1949, Notes for an Autobiography Thermodynamics is a funny subject. The first time you go through it, you don’t understand it at all. The second time you go through it, you think you understand it, except for one or two points. The third time you go through it, you know you don’t understand it, but by that time you are so used to the subject, it doesn’t bother you anymore. attributed to Arnold Sommerfeld, around 1940 Translation from French to English: Greg Cabailh4 1email: [email protected] 2email: [email protected] 3email: [email protected] 4email: [email protected] 2 Contents Preface and bibliography 7 1 Review of basic concepts 9 1.1 Thermodynamic systems . .9 1.2 Thermodynamic equilibrium . 10 1.3 Thermodynamic variables . 10 1.4 Transformations . 12 1.5 Internal energy U ................................. 13 1.6 Pressure p ..................................... 16 1.7 Temperature T .................................. 18 2 Energy transfer 19 2.1 Energy conservation, work, heat . 19 2.2 Some examples of energy exchange through work . -

Chbe 11: Chemical Engineering Thermodynamics
ChBE 11: Chemical Engineering Thermodynamics Andrew Rosen October 25, 2018 Contents 1 Measured Thermodynamic Properties and Other Basic Concepts 4 1.1 Preliminary Concepts - The Language of Thermo . .4 1.2 Measured Thermodynamic Properties . .4 1.3 Equilibrium . .4 1.4 Independent and Dependent Thermodynamic Properties . .5 1.5 The P vT Surface and its Projections for Pure Substances . .5 1.6 Thermodynamic Property Tables . .5 1.7 Lever Rule . .5 2 The First Law of Thermodynamics 5 2.1 The First Law of Thermodynamics . .5 2.2 Reversible and Irreversible Processes . .6 2.3 The First Law of Thermodynamics for Closed Systems . .6 2.4 The First Law of Thermodynamics for Open Systems . .6 2.5 Thermochemical Data for U and H ..........................................7 2.6 Open-System Steady State Energy Balance on Process Equipment . .8 2.7 Thermodynamics and the Carnot Cycle . .8 2.8 Summary of Calculating First Law Quantities at Steady-Sate when Shaft-Work, Kinetic Energy, and Potential Energy are Ignored for an Ideal Gas . .9 2.8.1 Constant Pressure (Isobaric) Heating . .9 2.8.2 Constant Volume (Isochoric) Heating . .9 2.8.3 Adiabatic Flame Temperature (Isobaric/Adiabatic) . .9 2.8.4 Reversible Isothermal Process in a Perfect Gas . .9 2.8.5 Reversible Adiabatic Process in a Perfect Gas with Constant Heat Capacity . 10 2.8.6 Adiabatic Expansion of a Perfect Gas into a Vacuum . 10 2.8.7 Reversible Phase Change at Constant T and P ............................... 10 3 Entropy and the Second Law of Thermodynamics 11 3.1 Directionality of Processes/Spontaneity . -

MODELING and SIMULATION of SUPERSONIC GAS DEHYDRATION USING JOULE THOMSON VALVE by Ho Chia Ming 7720 Dissertation Submitted in P
MODELING AND SIMULATION OF SUPERSONIC GAS DEHYDRATION USING JOULE THOMSON VALVE by Ho Chia Ming 7720 Dissertation submitted in partial fulfillment of the requirements for the Bachelor of Engineering (Hons) (Chemical Engineering) JULY 2009 Universiti Teknologi PETRONAS Bandar Seri Iskandar 31750 Tronoh I CERTIFICATION OF APPROVAL MODELING AND SIMULATION OF SUPERSONIC GAS DEHYDRATION USING JOULE THOMSON VALVE by Ho Chia Ming A project dissertation submitted to the Chemical Engineering Programme Universiti Teknologi PETRONAS in partial fulfilment of the requirement for the Bachelor of Engineering (Hons) (Chemical Engineering) Approved by, _________________________ (DR. LAU KOK KEONG) UNIVERSITI TEKNOLOGI PETRONAS TRONOH, PERAK JULY 2009 II CERTIFICATION OF ORIGINALITY This is to certify that I am responsible for the work submitted in this project, that the original work is my own except as specified in the references and acknowledgements, and that the original work contained herein have not been undertaken or done by unspecified sources or persons. _____________________________________ (HO CHIA MING) III ABSTRACT Entitled “Supersonic gas dehydration in pipeline using Joule Thomson Valve”, this project ultimately aims to introduce a new technology, Joule Thomson valve to separate water from natural gas (from reservoir) using supersonic flow and joule Thomson cooling effect. Due to time and financial constraints, this project is only being done in simulation, not in term of experiment. Gas dehydration in pipeline is essential because water content in the gas system could cause large pressure drop and corrosions that will be enhanced by the presence of H2S and CO2 typically associated in sour gas. The current technologies used are membrane and absorption separation technique.