Of Britain and Ireland Part 6 Provisional Atlas of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
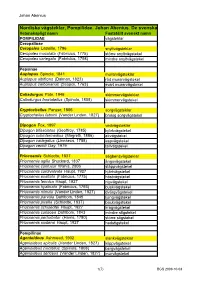
Nordiska Vägsteklar, Pompilidae. Johan Abenius. De Svenska
Johan Abenius Nordiska vägsteklar, Pompilidae. Johan Abenius. De svenska namnen fastställda av Kommittén för svenska djurnamn 100825 Vetenskapligt namn Fastställt svenskt namn POMPILIDAE vägsteklar Ceropalinae Ceropales Latreille, 1796 snyltvägsteklar Ceropales maculata (Fabricius, 1775) större snyltvägstekel Ceropales variegata (Fabricius, 1798) mindre snyltvägstekel Pepsinae Auplopus Spinola, 1841 murarvägsteklar Auplopus albifrons (Dalman, 1823) röd murarvägstekel Auplopus carbonarius (Scopoli, 1763) svart murarvägstekel Caliadurgus Pate, 1946 skimmervägsteklar Caliadurgus fasciatellus (Spinola, 1808) skimmervägstekel Cryptocheilus Panzer, 1806 sorgvägsteklar Cryptocheilus fabricii (Vander Linden, 1827) brokig sorgvägstekel Dipogon Fox, 1897 vedvägsteklar Dipogon bifasciatus (Geoffroy, 1785) björkvägstekel Dipogon subintermedius (Magretti, 1886) ekvägstekel Dipogon variegatus (Linnaeus, 1758) aspvägstekel Dipogon vechti Day, 1979 tallvägstekel Priocnemis Schiødte, 1837 sågbensvägsteklar Priocnemis agilis Shuckard, 1837 ängsvägstekel Priocnemis confusor Wahis, 2006 stäppvägstekel Priocnemis cordivalvata Haupt, 1927 hjärtvägstekel Priocnemis exaltata (Fabricius, 1775) höstvägstekel Priocnemis fennica Haupt, 1927 nipvägstekel Priocnemis hyalinata (Fabricius, 1793) buskvägstekel Priocnemis minuta (Vander Linden, 1827) dvärgvägstekel Priocnemis parvula Dahlbom, 1845 ljungvägstekel Priocnemis pusilla (Schiødte, 1837) backvägstekel Priocnemis schioedtei Haupt, 1927 kragvägstekel Priocnemis coriacea Dahlbom, 1843 mindre stigstekel Priocnemis -

Hymenoptera, Apoidea: Crabronidae) in Southern Iran
Number 303: 1-18 ISSN 1026-051X December 2015 hppt/urn:lsid:zoobank.org:pub:72563560-1CE7-457B-8BB1-593EEA1179EF NEW DATA ON THE DIGGER WASPS (HYMENOPTERA, APOIDEA: CRABRONIDAE) IN SOUTHERN IRAN Sh. Rezaei, M. Fallahzadeh* Department of Entomology, Jahrom branch, Islamic Azad University, Jahrom, Iran. *Corresponding author, E-mail: [email protected] The data on digger wasp (Hymenoptera: Crabronidae) collected using Malaise traps from Fars province in southern Iran are given. A total of 45 species and sub- species of 23 genera belonging to 5 subfamilies: Astatinae (three species in one genus), Bembicinae (five species in four genera), Crabroninae (26 species in 11 genera), Pemphredoninae (six species in five genera) and Philanthinae (five species in two genera) are herein listed. Of these, five species and one subspecies are newly recorded from Iran. In addition, seven species are new records for Fars province. The greatest percentage of specimens is that of the subfamily Crabroninae, with 63.6% captured material. KEY WORDS: Hymenoptera, wasps, Malaise trap, fauna, new records, Iran. Ш. Резаи, М. Фаллахзадэ. Новые данные о роющих осах (Hymenoptera, Apoidea: Crabronidae) южного Ирана // Дальневосточный энтомолог. 2015. N 303. С. 1-18. Приведены сведения о роющих осах (Hymenoptera: Crabronidae), собранных в провинции Фарс на юге Ирана. Список включает 45 видов и подвидов из 5 подсемейств: Astatinae (3 вида из 1 рода), Bembicinae (5 виддов из 4 родов), Crabroninae (26 видов из 11 родов), Pemphredoninae (6 видов из 5 родов) и 1 Philanthinae (5 видов из 2 родов). Из них 5 видов и 1 подвид впервые указы- ваются из Ирана. Кроме того, 7 видов впервые приводятся для провинции Фарс. -

A Comprehensive Guide to Insects of Britain & Ireland
a comprehensive guide to insects of Britain & ireland To be launched Spring 2014. order your copy now and save over £7.50! Offer endS 31 March 2014 Special expected list price: £27.50 Pre-Publication special price: £19.95 offer you save: £7.50 A comprehensive guide to Insects of Britain & irelAnd by Paul D. Brock Special expected list price: £27.50 * Scientific Associate of the Pre-Publication special price: £19.95 Natural History Museum, offer you save: £7.50 London, and author of the acclaimed ‘Insects of the New Forest’ full colour photographs throughout, with fully comprehensive sections on all insect 2 Ants, bees and wasps Subfamily Andreninae Ants, bees and wasps 3 Andrena species form the majority of this large subfamily of small to large, mining (soil-nesting) bees; very few groups, including flies, bees and wasps nest communally. There are sometimes several species with similar appearance, thus care is needed in identification. Many have a single brood, but identification of others with two broods is so by seasonal variation. In a few species, giant males occur, with large heads and mandibles. metimes complicated species and a few Sphecodes species are cleptoparasites and parasitic flies are often seen around Colourful nests where it is fascinating to watch their behaviour. A selection of species in this popular genus is include Nomada widespread, some are very local. ISBN 978-1-874357-58-2 d; although Andrena angustior Body length: 8–11 mm. Small, distinguished by the long marginal area on 2nd tergite. Cleptoparasite probably Nomada fabriciana Flexibound, 195 × 135mm, around 500pp woodlands, meadows and sometimes heaths. -

Towards Simultaneous Analysis of Morphological and Molecular Data in Hymenoptera
Towards simultaneous analysis of morphological and molecular data in Hymenoptera JAMES M. CARPENTER &WARD C. WHEELER Accepted 5 January 1999 Carpenter, J. M. & W. C. Wheeler. (1999). Towards simultaneous analysis of molecular and morphological data in Hymenoptera. Ð Zoologica Scripta 28, 251±260. Principles and methods of simultaneous analysis in cladistics are reviewed, and the first, preliminary, analysis of combined molecular and morphological data on higher level relationships in Hymenoptera is presented to exemplify these principles. The morphological data from Ronquist et al. (in press) matrix, derived from the character diagnoses of the phylogenetic tree of Rasnitsyn (1988), are combined with new molecular data for representatives of 10 superfamilies of Hymenoptera by means of optimization alignment. The resulting cladogram supports Apocrita and Aculeata as groups, and the superfamly Chrysidoidea, but not Chalcidoidea, Evanioidea, Vespoidea and Apoidea. James M. Carpenter, Department of Entomology, and Ward C. Wheeler, Department of Invertebrates, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024, U SA. E-mail: [email protected] Introduction of consensus techniques to the results of independent Investigation of the higher-level phylogeny of Hymenoptera analysis of multiple data sets, as for example in so-called is at a very early stage. Although cladistic analysis was ®rst `phylogenetic supertrees' (Sanderson et al. 1998), does not applied more than 30 years ago, in an investigation of the measure the strength of evidence supporting results from ovipositor by Oeser (1961), a comprehensive analysis of all the different data sources Ð in addition to other draw- the major lineages remains to be done. -

Wisconsin Bee Identification Guide
WisconsinWisconsin BeeBee IdentificationIdentification GuideGuide Developed by Patrick Liesch, Christy Stewart, and Christine Wen Honey Bee (Apis mellifera) The honey bee is perhaps our best-known pollinator. Honey bees are not native to North America and were brought over with early settlers. Honey bees are mid-sized bees (~ ½ inch long) and have brownish bodies with bands of pale hairs on the abdomen. Honey bees are unique with their social behavior, living together year-round as a colony consisting of thousands of individuals. Honey bees forage on a wide variety of plants and their colonies can be useful in agricultural settings for their pollination services. Honey bees are our only bee that produces honey, which they use as a food source for the colony during the winter months. In many cases, the honey bees you encounter may be from a local beekeeper’s hive. Occasionally, wild honey bee colonies can become established in cavities in hollow trees and similar settings. Photo by Christy Stewart Bumble bees (Bombus sp.) Bumble bees are some of our most recognizable bees. They are amongst our largest bees and can be close to 1 inch long, although many species are between ½ inch and ¾ inch long. There are ~20 species of bumble bees in Wisconsin and most have a robust, fuzzy appearance. Bumble bees tend to be very hairy and have black bodies with patches of yellow or orange depending on the species. Bumble bees are a type of social bee Bombus rufocinctus and live in small colonies consisting of dozens to a few hundred workers. Photo by Christy Stewart Their nests tend to be constructed in preexisting underground cavities, such as former chipmunk or rabbit burrows. -

Millichope Park and Estate Invertebrate Survey 2020
Millichope Park and Estate Invertebrate survey 2020 (Coleoptera, Diptera and Aculeate Hymenoptera) Nigel Jones & Dr. Caroline Uff Shropshire Entomology Services CONTENTS Summary 3 Introduction ……………………………………………………….. 3 Methodology …………………………………………………….. 4 Results ………………………………………………………………. 5 Coleoptera – Beeetles 5 Method ……………………………………………………………. 6 Results ……………………………………………………………. 6 Analysis of saproxylic Coleoptera ……………………. 7 Conclusion ………………………………………………………. 8 Diptera and aculeate Hymenoptera – true flies, bees, wasps ants 8 Diptera 8 Method …………………………………………………………… 9 Results ……………………………………………………………. 9 Aculeate Hymenoptera 9 Method …………………………………………………………… 9 Results …………………………………………………………….. 9 Analysis of Diptera and aculeate Hymenoptera … 10 Conclusion Diptera and aculeate Hymenoptera .. 11 Other species ……………………………………………………. 12 Wetland fauna ………………………………………………….. 12 Table 2 Key Coleoptera species ………………………… 13 Table 3 Key Diptera species ……………………………… 18 Table 4 Key aculeate Hymenoptera species ……… 21 Bibliography and references 22 Appendix 1 Conservation designations …………….. 24 Appendix 2 ………………………………………………………… 25 2 SUMMARY During 2020, 811 invertebrate species (mainly beetles, true-flies, bees, wasps and ants) were recorded from Millichope Park and a small area of adjoining arable estate. The park’s saproxylic beetle fauna, associated with dead wood and veteran trees, can be considered as nationally important. True flies associated with decaying wood add further significant species to the site’s saproxylic fauna. There is also a strong -

Seasonal and Spatial Patterns of Mortality and Sex Ratio in the Alfalfa
Seasonal and spatial patterns of mortality and sex ratio in the alfalfa leafcutting bee, Megachile rotundata (F.) by Ruth Pettinga ONeil A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Entomology Montana State University © Copyright by Ruth Pettinga ONeil (2004) Abstract: Nests from five seed alfalfa sites of the alfalfa leafcutting bee Megachile rotundata (F.) were monitored over the duration of the nesting season in 2000 and 2001, from early July through late August. Cells containing progeny of known age and known position within the nest were subsequently analyzed for five commonly encountered categories of pre-diapause mortality in this species. Chalkbrood and pollen ball had the strongest seasonal relationships of mortality factors studied. Chalkbrood incidence was highest in early-produced cells. Pollen ball was higher in late-season cells. Chalkbrood, parasitism by the chalcid Pteromalus venustus, and death of older larvae and prepupae , due to unknown source(s) exhibited the strongest cell-position relationships. Both chalkbrood and parasitoid incidence were highest in the inner portions of nests. The “unknown” category of mortality was highest in outer portions of nests. Sex ratio was determined for a subset of progeny reared to adulthood. The ratio of females to males is highest in cells in inner nest positions. Sex ratio is female-biased very early in the nesting season, when all cells being provisioned are the inner cells of nests, due to the strong positional effect on sex ratio. SEASONAL AND SPATIAL PATTERNS OF MORTALITY AND SEX RATIO IN THE ALFALFA LEAFCUTTING BEE, Megachile rotundata (F.) by . -

Final Report 1
Sand pit for Biodiversity at Cep II quarry Researcher: Klára Řehounková Research group: Petr Bogusch, David Boukal, Milan Boukal, Lukáš Čížek, František Grycz, Petr Hesoun, Kamila Lencová, Anna Lepšová, Jan Máca, Pavel Marhoul, Klára Řehounková, Jiří Řehounek, Lenka Schmidtmayerová, Robert Tropek Březen – září 2012 Abstract We compared the effect of restoration status (technical reclamation, spontaneous succession, disturbed succession) on the communities of vascular plants and assemblages of arthropods in CEP II sand pit (T řebo ňsko region, SW part of the Czech Republic) to evaluate their biodiversity and conservation potential. We also studied the experimental restoration of psammophytic grasslands to compare the impact of two near-natural restoration methods (spontaneous and assisted succession) to establishment of target species. The sand pit comprises stages of 2 to 30 years since site abandonment with moisture gradient from wet to dry habitats. In all studied groups, i.e. vascular pants and arthropods, open spontaneously revegetated sites continuously disturbed by intensive recreation activities hosted the largest proportion of target and endangered species which occurred less in the more closed spontaneously revegetated sites and which were nearly absent in technically reclaimed sites. Out results provide clear evidence that the mosaics of spontaneously established forests habitats and open sand habitats are the most valuable stands from the conservation point of view. It has been documented that no expensive technical reclamations are needed to restore post-mining sites which can serve as secondary habitats for many endangered and declining species. The experimental restoration of rare and endangered plant communities seems to be efficient and promising method for a future large-scale restoration projects in abandoned sand pits. -

Asiatische Halictidae, 3. Die Artengruppe Der Lasioglossum Carinate-/Jvy/Ae«S (Insecta: Hymenoptera: Apoidea: Halictidae: Halictinae)
© Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Linzer biol. Beitr. 27/2 525-652 29.12.1995 Asiatische Halictidae, 3. Die Artengruppe der Lasioglossum carinate-/jvy/ae«s (Insecta: Hymenoptera: Apoidea: Halictidae: Halictinae) A. W. EBMER Abstract: The palearctic species of the big species group Lasioglossum carinate- Evylaeus according to SAKAGAMI and other authors is presented. For the first time the numerous palearctic species are sorted into species groups. New taxa described are: Lasioglossum (Evylaeus) albipes villosum 9 (Rußland, Primorskij kraj), alexandrirtum 3 9 (Turkmenien, Kirgisien), eschaton 9 (Tadjikistan, Kirgisien, Usbekien, Turkmenien), anthrax 9 (China, Yunnan). Until recently unknown sexes of the following taxa are described for the first time: La- sioglossum {Evylaeus) reinigi EBMER 1978 3, nipponense (HIRASHIMA 1953) 3, salebrosum (BLÜTHGEN 1943) 9, apristum (VACHAL 1903) 3, epipygiale epipygiale (BLÜTHGEN 1924) 9, israelense EBMER 1974 3, calileps (BLÜTHGEN 1926) 9, sim- laense (CAMERON 1909) $, mesoviride EBMER 1974 3, leucopymatum (DALLA TORRE 1896) d\ edessae EBMER 1974 9, nursei (BLÜTHGEN 1926) 3, himalayense (BlNGHAM 1898) 3, perihirtulum (COCKERELL 1937) 3, suppressum EBMER 1983 9, oppositum (SMITH 1875) 3, funebre (CAMERON 1896) 9. Type specimens from A. Fedoenko's expedition to central Asia (1868-1871) have been studied and informations about localities are recorded which are not common knowledge to today entomologists. Einleitung Anlaß fur diese Publikation sind neue, umfangreichere Aufsammlungen vor allem aus Zentralasien. Schon vor vielen Jahren schenkte mir Dr. Wilhelm Grünwaldt, München, Aufsammlungen vor allem aus Turkmenien, aber auch aus den anderen, heute neuen Staaten Zentralasiens. Im Jahr 1990 schenkte mit Prof. Joachim Oehlke seine Auf- sammlungen aus Turkmenien sowie aus dem südlichen Kaukasus. -

Yorkhill Green Spaces Wildlife Species List
Yorkhill Green Spaces Wildlife Species List April 2021 update Yorkhill Green Spaces Species list Draft list of animals, plants, fungi, mosses and lichens recorded from Yorkhill, Glasgow. Main sites: Yorkhill Park, Overnewton Park and Kelvinhaugh Park (AKA Cherry Park). Other recorded sites: bank of River Kelvin at Bunhouse Rd/ Old Dumbarton Rd, Clyde Expressway path, casual records from streets and gardens in Yorkhill. Species total: 711 Vertebrates: Amhibians:1, Birds: 57, Fish: 7, Mammals (wild): 15 Invertebrates: Amphipods: 1, Ants: 3, Bees: 26, Beetles: 21, Butterflies: 11, Caddisflies: 2, Centipedes: 3, Earthworms: 2, Earwig: 1, Flatworms: 1, Flies: 61, Grasshoppers: 1, Harvestmen: 2, Lacewings: 2, Mayflies: 2, Mites: 4, Millipedes: 3, Moths: 149, True bugs: 13, Slugs & snails: 21, Spiders: 14, Springtails: 2, Wasps: 13, Woodlice: 5 Plants: Flowering plants: 174, Ferns: 5, Grasses: 13, Horsetail: 1, Liverworts: 7, Mosses:17, Trees: 19 Fungi and lichens: Fungi: 24, Lichens: 10 Conservation Status: NameSBL - Scottish Biodiversity List Priority Species Birds of Conservation Concern - Red List, Amber List Last Common name Species Taxon Record Common toad Bufo bufo amphiban 2012 Australian landhopper Arcitalitrus dorrieni amphipod 2021 Black garden ant Lasius niger ant 2020 Red ant Myrmica rubra ant 2021 Red ant Myrmica ruginodis ant 2014 Buff-tailed bumblebee Bombus terrestris bee 2021 Garden bumblebee Bombus hortorum bee 2020 Tree bumblebee Bombus hypnorum bee 2021 Heath bumblebee Bombus jonellus bee 2020 Red-tailed bumblebee Bombus -

Bees and Wasps of the East Sussex South Downs
A SURVEY OF THE BEES AND WASPS OF FIFTEEN CHALK GRASSLAND AND CHALK HEATH SITES WITHIN THE EAST SUSSEX SOUTH DOWNS Steven Falk, 2011 A SURVEY OF THE BEES AND WASPS OF FIFTEEN CHALK GRASSLAND AND CHALK HEATH SITES WITHIN THE EAST SUSSEX SOUTH DOWNS Steven Falk, 2011 Abstract For six years between 2003 and 2008, over 100 site visits were made to fifteen chalk grassland and chalk heath sites within the South Downs of Vice-county 14 (East Sussex). This produced a list of 227 bee and wasp species and revealed the comparative frequency of different species, the comparative richness of different sites and provided a basic insight into how many of the species interact with the South Downs at a site and landscape level. The study revealed that, in addition to the character of the semi-natural grasslands present, the bee and wasp fauna is also influenced by the more intensively-managed agricultural landscapes of the Downs, with many species taking advantage of blossoming hedge shrubs, flowery fallow fields, flowery arable field margins, flowering crops such as Rape, plus plants such as buttercups, thistles and dandelions within relatively improved pasture. Some very rare species were encountered, notably the bee Halictus eurygnathus Blüthgen which had not been seen in Britain since 1946. This was eventually recorded at seven sites and was associated with an abundance of Greater Knapweed. The very rare bees Anthophora retusa (Linnaeus) and Andrena niveata Friese were also observed foraging on several dates during their flight periods, providing a better insight into their ecology and conservation requirements. -

Bee-Plant Networks: Structure, Dynamics and the Metacommunity Concept
Ber. d. Reinh.-Tüxen-Ges. 28, 23-40. Hannover 2016 Bee-plant networks: structure, dynamics and the metacommunity concept – Anselm Kratochwil und Sabrina Krausch, Osnabrück – Abstract Wild bees play an important role within pollinator-plant webs. The structure of such net- works is influenced by the regional species pool. After special filtering processes an actual pool will be established. According to the results of model studies these processes can be elu- cidated, especially for dry sandy grassland habitats. After restoration of specific plant com- munities (which had been developed mainly by inoculation of plant material) in a sandy area which was not or hardly populated by bees before the colonization process of bees proceeded very quickly. Foraging and nesting resources are triggering the bee species composition. Dis- persal and genetic bottlenecks seem to play a minor role. Functional aspects (e.g. number of generalists, specialists and cleptoparasites; body-size distributions) of the bee communities show that ecosystem stabilizing factors may be restored rapidly. Higher wild-bee diversity and higher numbers of specialized species were found at drier plots, e.g. communities of Koelerio-Corynephoretea and Festuco-Brometea. Bee-plant webs are highly complex systems and combine elements of nestedness, modularization and gradients. Beside structural com- plexity bee-plant networks can be characterized as dynamic systems. This is shown by using the metacommunity concept. Zusammenfassung: Wildbienen-Pflanzenarten-Netzwerke: Struktur, Dynamik und das Metacommunity-Konzept. Wildbienen spielen eine wichtige Rolle innerhalb von Bestäuber-Pflanzen-Netzwerken. Ihre Struktur wird vom jeweiligen regionalen Artenpool bestimmt. Nach spezifischen Filter- prozessen bildet sich ein aktueller Artenpool.