Effect of Transcription Factor ZBTB20 on Mouse Pituitary Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 T + is online at: average * The Journal of Immunology , 34 of which you can access for free at: 2016; 197:1477-1488; Prepublished online 1 July from submission to initial decision 4 weeks from acceptance to publication 2016; doi: 10.4049/jimmunol.1600589 http://www.jimmunol.org/content/197/4/1477 Molecular Profile of Tumor-Specific CD8 Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. Waugh, Sonia M. Leach, Brandon L. Moore, Tullia C. Bruno, Jonathan D. Buhrman and Jill E. Slansky J Immunol cites 95 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2016/07/01/jimmunol.160058 9.DCSupplemental This article http://www.jimmunol.org/content/197/4/1477.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 25, 2021. The Journal of Immunology Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. -

Health-Relevant Phenotypes in the Offspring of Mice Given CAR Activators Prior to Pregnancy S
Supplemental material to this article can be found at: http://dmd.aspetjournals.org/content/suppl/2018/08/28/dmd.118.082925.DC1 1521-009X/46/11/1827–1835$35.00 https://doi.org/10.1124/dmd.118.082925 DRUG METABOLISM AND DISPOSITION Drug Metab Dispos 46:1827–1835, November 2018 Copyright ª 2018 by The American Society for Pharmacology and Experimental Therapeutics Health-Relevant Phenotypes in the Offspring of Mice Given CAR Activators Prior to Pregnancy s Karin Dietrich, Jan Baumgart, Leonid Eshkind, Lea Reuter, Ute Gödtel-Armbrust, Elke Butt, Michael Musheev, Federico Marini, Piyush More, Tanja Grosser, Christof Niehrs, Leszek Wojnowski, and Marianne Mathäs Department of Pharmacology (K.D., L.R., U.G.-A., P.M., T.G., L.W., M.Ma.) and Institute of Medical Biostatistics, Epidemiology and Informatics (F.M.), University Medical Center Mainz, Mainz, Germany; Translational Animal Research Center (J.B., L.E.), University Medical Center, Johannes Gutenberg University Mainz, Mainz, Germany; Institute of Experimental Biomedicine II, University Hospital Würzburg, Würzburg, Germany (E.B.); Institute of Molecular Biology, Mainz, Germany (M.Mu., C.N.); and Division of Molecular Embryology, German Cancer Research Center (DKFZ-ZMBH Alliance), Heidelberg, Germany (C.N.) Received June 6, 2018; accepted August 22, 2018 Downloaded from ABSTRACT Hepatic induction in response to drugs and environmental chem- analyses. The important environmental pollutant PCB153 acti- icals affects drug therapies and energy metabolism. We investigated vated CAR in the F1 generation in a manner similar to TCPOBOP. whether the induction is transmitted to the offspring. We injected Our findings indicate that chemicals accumulating and persisting 3-day- and 6-week-old F0 female mice with TCPOBOP, an activator in adipose tissue may exert liver-mediated, health-relevant effects dmd.aspetjournals.org of the nuclear receptor constitutive androstane receptor (CAR, on F1 offspring simply via physical transmission in milk. -
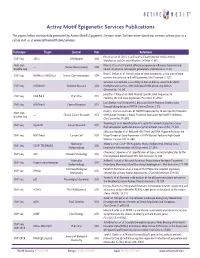
Active Motif Epigenetic Services Publications the Papers Below Contain Data Generated by Active Motif’S Epigenetic Services Team
Active Motif Epigenetic Services Publications The papers below contain data generated by Active Motif’s Epigenetic Services team. To learn more about our services, please give us a call or visit us at www.activemotif.com/services. Technique Target Journal Year Reference Erin Stashi et al. SRC-2 Is an Essential Coactivator for Orchestrating ChIP-Seq SRC-2 Cell Reports 2014 Metabolism and Circadian Rhythm. Cell Rep. 6: 663 Next-Gen Brian G. Dias et al. Parental olfactory experience influences behavior and — Nature Neuroscience 2014 Bisulfite Seq neural structure in subsequent generations. Nat Neurosci. 17: 89 Brian C. Belyea et al. Identification of renin progenitors in the mouse bone ChIP-Seq H3K4me3, H3K27me3 Nature Communications 2014 marrow that give rise to B-cell leukaemia. Nat Commun. 5: 3273 Variation in chromatin accessibility in human kidney cancer links H3K36 ChIP-Seq H3K36me3 Genome Research 2014 methyltransferase loss with widespread RNA processing defects. Genome Res. 24: 241 Jonathan P. Riley et al. PARP-14 Binds Specific DNA Sequences to ChIP-Seq RNA Pol II PLoS One 2013 Promote Th2 Cell Gene Expression. PLoS One. 8: e83127 Lynn Bjerke et al. Histone H3.3 Mutations Drive Pediatric Glioblastoma ChIP-Seq H3K36me3 Cancer Discovery 2013 through Upregulation of MYCN. Cancer Discov. 3: 512 David S. Shames et al. Loss of NAPRT1 Expression by Tumor-Specific Promoter Next-Gen — Clinical Cancer Research 2013 Methylation Provides a Novel Predictive Biomarker for NAMPT Inhibitors. Bisulfite Seq Clin Cancer Res. 19: 6912 Xiaoming Ju et al. Identification of a cyclin D1 network in prostate cancer ChIP-Seq Cyclin D1 Cancer Research 2013 that antagonizes epithelial-mesenchymal restraint. -

Transcriptional and Post-Transcriptional Regulation of ATP-Binding Cassette Transporter Expression
Transcriptional and Post-transcriptional Regulation of ATP-binding Cassette Transporter Expression by Aparna Chhibber DISSERTATION Submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHILOSOPHY in Pharmaceutical Sciences and Pbarmacogenomies in the Copyright 2014 by Aparna Chhibber ii Acknowledgements First and foremost, I would like to thank my advisor, Dr. Deanna Kroetz. More than just a research advisor, Deanna has clearly made it a priority to guide her students to become better scientists, and I am grateful for the countless hours she has spent editing papers, developing presentations, discussing research, and so much more. I would not have made it this far without her support and guidance. My thesis committee has provided valuable advice through the years. Dr. Nadav Ahituv in particular has been a source of support from my first year in the graduate program as my academic advisor, qualifying exam committee chair, and finally thesis committee member. Dr. Kathy Giacomini graciously stepped in as a member of my thesis committee in my 3rd year, and Dr. Steven Brenner provided valuable input as thesis committee member in my 2nd year. My labmates over the past five years have been incredible colleagues and friends. Dr. Svetlana Markova first welcomed me into the lab and taught me numerous laboratory techniques, and has always been willing to act as a sounding board. Michael Martin has been my partner-in-crime in the lab from the beginning, and has made my days in lab fly by. Dr. Yingmei Lui has made the lab run smoothly, and has always been willing to jump in to help me at a moment’s notice. -

Epigenetic Services Citations
Active Motif Epigenetic Services Publications The papers below contain data generated by Active Motif’s Epigenetic Services team. To learn more about our services, please give us a call or visit us at www.activemotif.com/services. Technique Target Journal Year Reference Justin C. Boucher et al. CD28 Costimulatory Domain- ATAC-Seq, Cancer Immunol. Targeted Mutations Enhance Chimeric Antigen Receptor — 2021 RNA-Seq Res. T-cell Function. Cancer Immunol. Res. doi: 10.1158/2326- 6066.CIR-20-0253. Satvik Mareedu et al. Sarcolipin haploinsufficiency Am. J. Physiol. prevents dystrophic cardiomyopathy in mdx mice. RNA-Seq — Heart Circ. 2021 Am J Physiol Heart Circ Physiol. doi: 10.1152/ Physiol. ajpheart.00601.2020. Gabi Schutzius et al. BET bromodomain inhibitors regulate Nature Chemical ChIP-Seq BRD4 2021 keratinocyte plasticity. Nat. Chem. Biol. doi: 10.1038/ Biology s41589-020-00716-z. Siyun Wang et al. cMET promotes metastasis and ChIP-qPCR FOXO3 J. Cell Physiol. 2021 epithelial-mesenchymal transition in colorectal carcinoma by repressing RKIP. J. Cell Physiol. doi: 10.1002/jcp.30142. Sonia Iyer et al. Genetically Defined Syngeneic Mouse Models of Ovarian Cancer as Tools for the Discovery of ATAC-Seq — Cancer Discovery 2021 Combination Immunotherapy. Cancer Discov. doi: doi: 10.1158/2159-8290 Vinod Krishna et al. Integration of the Transcriptome and Genome-Wide Landscape of BRD2 and BRD4 Binding BRD2, BRD4, RNA Motifs Identifies Key Superenhancer Genes and Reveals ChIP-Seq J. Immunol. 2021 Pol II the Mechanism of Bet Inhibitor Action in Rheumatoid Arthritis Synovial Fibroblasts. J. Immunol. doi: doi: 10.4049/ jimmunol.2000286. Daniel Haag et al. -

Integrative Bulk and Single-Cell Profiling of Premanufacture T-Cell Populations Reveals Factors Mediating Long-Term Persistence of CAR T-Cell Therapy
Published OnlineFirst April 5, 2021; DOI: 10.1158/2159-8290.CD-20-1677 RESEARCH ARTICLE Integrative Bulk and Single-Cell Profiling of Premanufacture T-cell Populations Reveals Factors Mediating Long-Term Persistence of CAR T-cell Therapy Gregory M. Chen1, Changya Chen2,3, Rajat K. Das2, Peng Gao2, Chia-Hui Chen2, Shovik Bandyopadhyay4, Yang-Yang Ding2,5, Yasin Uzun2,3, Wenbao Yu2, Qin Zhu1, Regina M. Myers2, Stephan A. Grupp2,5, David M. Barrett2,5, and Kai Tan2,3,5 Downloaded from cancerdiscovery.aacrjournals.org on October 1, 2021. © 2021 American Association for Cancer Research. Published OnlineFirst April 5, 2021; DOI: 10.1158/2159-8290.CD-20-1677 ABSTRACT The adoptive transfer of chimeric antigen receptor (CAR) T cells represents a breakthrough in clinical oncology, yet both between- and within-patient differences in autologously derived T cells are a major contributor to therapy failure. To interrogate the molecular determinants of clinical CAR T-cell persistence, we extensively characterized the premanufacture T cells of 71 patients with B-cell malignancies on trial to receive anti-CD19 CAR T-cell therapy. We performed RNA-sequencing analysis on sorted T-cell subsets from all 71 patients, followed by paired Cellular Indexing of Transcriptomes and Epitopes (CITE) sequencing and single-cell assay for transposase-accessible chromatin sequencing (scATAC-seq) on T cells from six of these patients. We found that chronic IFN signaling regulated by IRF7 was associated with poor CAR T-cell persistence across T-cell subsets, and that the TCF7 regulon not only associates with the favorable naïve T-cell state, but is maintained in effector T cells among patients with long-term CAR T-cell persistence. -

WO 2019/089592 Al 09 May 2019 (09.05.2019) W 1 P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization III International Bureau (10) International Publication Number (43) International Publication Date WO 2019/089592 Al 09 May 2019 (09.05.2019) W 1 P O PCT (51) International Patent Classification: (72) Inventors; and A61K 35/12 (2015.01) C07K 16/30 (2006.01) (71) Applicants: JAN, Max [US/US]; c/o The Broad Institute, A61K 5/7 7 (2015.01) C07K 14/705 (2006.01) Inc., 45 1 Main Street, Cambridge, Massachusetts 02142 (US). SIEVERS, Quinlan [US/US]; c/o The Broad In¬ (21) International Application Number: stitute, Inc., 451 Main Street, Cambridge, Massachusetts PCT/US2018/058210 02142 (US). EBERT, Benjamin [US/US]; c/o The Broad (22) International Filing Date: Institute, Inc., 451 Main Street, Cambridge, Massachusetts 30 October 2018 (30. 10.2018) 02142 (US). MAUS, Marcela [US/US]; c/o The Broad In¬ stitute, Inc., 451 Main Street, Cambridge, Massachusetts (25) Filing Language: English 02142 (US). (26) Publication Language: English (74) Agent: COWLES, Christopher R. et al; Burns & Levin- (30) Priority Data: son, LLP, 125 Summer Street, Boston, Massachusetts 62/579,454 3 1 October 2017 (3 1. 10.2017) U S 021 10 (US). 62/633,725 22 February 2018 (22.02.2018) U S (81) Designated States (unless otherwise indicated, for every (71) Applicants: THE GENERAL HOSPITAL CORPO¬ kind of national protection available): AE, AG, AL, AM, RATION [US/US]; 55 Fruit Street, Boston, Massachu¬ AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, setts 021 14 (US) BRIGHAM & WOMEN'S HOSPITAL CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, [US/US]; 75 Francis Street, Boston, Massachusetts 021 15 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, (US) PRESIDENT AND FELLOWS OF HARVARD HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, COLLEGE [US/US]; 17 Quincy Street, Cambridge, Mass¬ KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, achusetts 02138 (US). -

Identification of Transcriptional Mechanisms Downstream of Nf1 Gene Defeciency in Malignant Peripheral Nerve Sheath Tumors Daochun Sun Wayne State University
Wayne State University DigitalCommons@WayneState Wayne State University Dissertations 1-1-2012 Identification of transcriptional mechanisms downstream of nf1 gene defeciency in malignant peripheral nerve sheath tumors Daochun Sun Wayne State University, Follow this and additional works at: http://digitalcommons.wayne.edu/oa_dissertations Recommended Citation Sun, Daochun, "Identification of transcriptional mechanisms downstream of nf1 gene defeciency in malignant peripheral nerve sheath tumors" (2012). Wayne State University Dissertations. Paper 558. This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. IDENTIFICATION OF TRANSCRIPTIONAL MECHANISMS DOWNSTREAM OF NF1 GENE DEFECIENCY IN MALIGNANT PERIPHERAL NERVE SHEATH TUMORS by DAOCHUN SUN DISSERTATION Submitted to the Graduate School of Wayne State University, Detroit, Michigan in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY 2012 MAJOR: MOLECULAR BIOLOGY AND GENETICS Approved by: _______________________________________ Advisor Date _______________________________________ _______________________________________ _______________________________________ © COPYRIGHT BY DAOCHUN SUN 2012 All Rights Reserved DEDICATION This work is dedicated to my parents and my wife Ze Zheng for their continuous support and understanding during the years of my education. I could not achieve my goal without them. ii ACKNOWLEDGMENTS I would like to express tremendous appreciation to my mentor, Dr. Michael Tainsky. His guidance and encouragement throughout this project made this dissertation come true. I would also like to thank my committee members, Dr. Raymond Mattingly and Dr. John Reiners Jr. for their sustained attention to this project during the monthly NF1 group meetings and committee meetings, Dr. -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -

Supplementary Material Computational Prediction of SARS
Supplementary_Material Computational prediction of SARS-CoV-2 encoded miRNAs and their putative host targets Sheet_1 List of potential stem-loop structures in SARS-CoV-2 genome as predicted by VMir. Rank Name Start Apex Size Score Window Count (Absolute) Direct Orientation 1 MD13 2801 2864 125 243.8 61 2 MD62 11234 11286 101 211.4 49 4 MD136 27666 27721 104 205.6 119 5 MD108 21131 21184 110 204.7 210 9 MD132 26743 26801 119 188.9 252 19 MD56 9797 9858 128 179.1 59 26 MD139 28196 28233 72 170.4 133 28 MD16 2934 2974 76 169.9 71 43 MD103 20002 20042 80 159.3 403 46 MD6 1489 1531 86 156.7 171 51 MD17 2981 3047 131 152.8 38 87 MD4 651 692 75 140.3 46 95 MD7 1810 1872 121 137.4 58 116 MD140 28217 28252 72 133.8 62 122 MD55 9712 9758 96 132.5 49 135 MD70 13171 13219 93 130.2 131 164 MD95 18782 18820 79 124.7 184 173 MD121 24086 24135 99 123.1 45 176 MD96 19046 19086 75 123.1 179 196 MD19 3197 3236 76 120.4 49 200 MD86 17048 17083 73 119.8 428 223 MD75 14534 14600 137 117 51 228 MD50 8824 8870 94 115.8 79 234 MD129 25598 25642 89 115.6 354 Reverse Orientation 6 MR61 19088 19132 88 197.8 271 10 MR72 23563 23636 148 188.8 286 11 MR11 3775 3844 136 185.1 116 12 MR94 29532 29582 94 184.6 271 15 MR43 14973 15028 109 183.9 226 27 MR14 4160 4206 89 170 241 34 MR35 11734 11792 111 164.2 37 52 MR5 1603 1652 89 152.7 118 53 MR57 18089 18132 101 152.7 139 94 MR8 2804 2864 122 137.4 38 107 MR58 18474 18508 72 134.9 237 117 MR16 4506 4540 72 133.8 311 120 MR34 10010 10048 82 132.7 245 133 MR7 2534 2578 90 130.4 75 146 MR79 24766 24808 75 127.9 59 150 MR65 21528 21576 99 127.4 83 180 MR60 19016 19049 70 122.5 72 187 MR51 16450 16482 75 121 363 190 MR80 25687 25734 96 120.6 75 198 MR64 21507 21544 70 120.3 35 206 MR41 14500 14542 84 119.2 94 218 MR84 26840 26894 108 117.6 94 Sheet_2 List of stable stem-loop structures based on MFE. -

Zinc Finger Protein ZBTB20 Promotes Toll-Like Receptor-Triggered Innate
Zinc finger protein ZBTB20 promotes toll-like receptor-triggered innate immune responses by repressing IκBα gene transcription Xingguang Liua,1, Peng Zhanga,1, Yan Baoa,1, Yanmei Hana, Yan Wangb, Qian Zhangc, Zhenzhen Zhana, Jun Mengd, Yingke Lia, Nan Lia, Weiping J. Zhangb,2, and Xuetao Caoa,c,d,2 aNational Key Laboratory of Medical Immunology and Institute of Immunology and bDepartment of Pathophysiology, Second Military Medical University, Shanghai 200433, China; cNational Key Laboratory of Molecular Biology and Department of Immunology, Chinese Academy of Medical Sciences, Beijing 100730, China; and dInstitute of Immunology, Zhejiang University School of Medicine, Hangzhou 310058, China Edited by Genhong Cheng, University of California, Los Angeles, CA, and accepted by the Editorial Board May 21, 2013 (received for review January 24, 2013) Toll-like receptor (TLR) signaling is critical in innate response signaling is tightly regulated to maintain the immunological against invading pathogens. However, the molecular mechanisms homeostasis. Previous studies have shown that the TLR pathway for full activation of TLR-triggered innate immunity need to be is positively or negatively regulated by various signaling mole- fully elucidated. The broad complex tramtrack bric-a-brac/poxvirus cules (7–9). However, the underlying molecular mechanism and zinc finger (BTB/POZ) family is a class of transcription factors through which TLR response is properly initiated and fully ac- involved in many biological processes. However, few BTB/POZ tivated still needs to be further clarified. proteins were reported to function in innate immune response. Zinc The broad complex tramtrack bric-a-brac/poxvirus and zinc fi finger and BTB domain-containing 20 (ZBTB20), a member of BTB/POZ nger (BTB/POZ) family is a class of nuclear DNA binding family, functions in neurogenesis and represses α-fetoprotein transcription factors that is involved in a variety of biological gene transcription in liver. -

Epigenetic Mechanisms Are Involved in the Oncogenic Properties of ZNF518B in Colorectal Cancer
Epigenetic mechanisms are involved in the oncogenic properties of ZNF518B in colorectal cancer Francisco Gimeno-Valiente, Ángela L. Riffo-Campos, Luis Torres, Noelia Tarazona, Valentina Gambardella, Andrés Cervantes, Gerardo López-Rodas, Luis Franco and Josefa Castillo SUPPLEMENTARY METHODS 1. Selection of genomic sequences for ChIP analysis To select the sequences for ChIP analysis in the five putative target genes, namely, PADI3, ZDHHC2, RGS4, EFNA5 and KAT2B, the genomic region corresponding to the gene was downloaded from Ensembl. Then, zoom was applied to see in detail the promoter, enhancers and regulatory sequences. The details for HCT116 cells were then recovered and the target sequences for factor binding examined. Obviously, there are not data for ZNF518B, but special attention was paid to the target sequences of other zinc-finger containing factors. Finally, the regions that may putatively bind ZNF518B were selected and primers defining amplicons spanning such sequences were searched out. Supplementary Figure S3 gives the location of the amplicons used in each gene. 2. Obtaining the raw data and generating the BAM files for in silico analysis of the effects of EHMT2 and EZH2 silencing The data of siEZH2 (SRR6384524), siG9a (SRR6384526) and siNon-target (SRR6384521) in HCT116 cell line, were downloaded from SRA (Bioproject PRJNA422822, https://www.ncbi. nlm.nih.gov/bioproject/), using SRA-tolkit (https://ncbi.github.io/sra-tools/). All data correspond to RNAseq single end. doBasics = TRUE doAll = FALSE $ fastq-dump -I --split-files SRR6384524 Data quality was checked using the software fastqc (https://www.bioinformatics.babraham. ac.uk /projects/fastqc/). The first low quality removing nucleotides were removed using FASTX- Toolkit (http://hannonlab.cshl.edu/fastxtoolkit/).